If a radical substitution reaction creates a chirality centre in the product, both the R and S enantiomers will be formed.
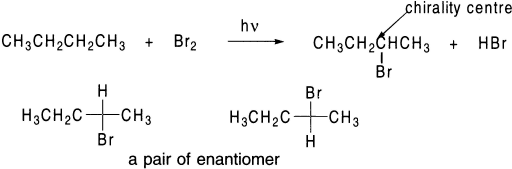
The carbon bearing the unpaired electron is sp2 hybridized, that is, the three groups to which it is bonded lie in a plane. The incoming halogen has equal access to both sides of the plane. As a result, both the R and S enantiomers are formed in equal amounts.
If the reactant already has a chirality centre, and the radical substitution reaction creates a second chirality centre, in such a case, a pair of diastereomers will be formed in unequal amounts. Diastereomers will be formed because the new chirality centre created in the product can have either the R or the S configuration, but the configuration of the chirality centre in the reactant will be unchanged in the product, because none of the bonds to that chirality centre is broken during the course of the reaction.
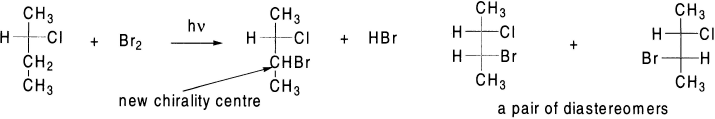
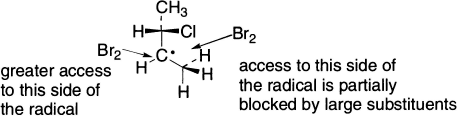
One of the diastereomers formed will be in greater amount than the other, because the incoming Br2 will have greater access to one side of the radical intermediate than to the other due to the presence of the original chirality centre.
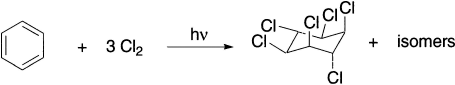
Benzenoid compounds can also react by addition with chlorine atoms, for example, the irradiation of benzene and chlorine gives a mixture of stereoisomeric hexachlorocyclohexanes.
The addition of deuterium bromide to both cis– and trans-2-butene proceeds in a stereospecific trans manner at low temperature. The cis-olefin yields threo and the trans gives the erythro bromide. Similarly, the addition of HBr to isomeric 2-bromo-2-butenes is stereospecific at low temperature and in excess of HBr. The stereospecificity decreases as the temperature of the reaction is increased. At room temperature, both olefins yield the same mixture of products. Goering and Larsen first suggested that two different conformations are involved as intermediates from cis– and trans-olefins. The life time of these two conformations is so short that they cannot interconvert prior to the chain transfer step, which takes place from the less hindered side. At room temperature, however, these can obtain equilibrium rapidly because of easy rotation about the C-C bond, which results in the same mixture of meso– and d,l-2,3-dibromobutanes. Another reason may be that the addition of bromine radical (Br·) to noncyclic olefins is often reversible and may lead to nonstereospecific products.
The second mechanism assumes a π-complex formation between olefin and HBr. A bromine atom then collides with the complex leading to its attachment and simultaneous breaking of the HBr bond, explaining the decrease in stereospecificity with rise in temperature.
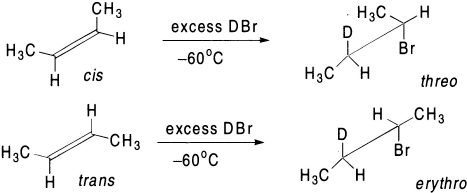
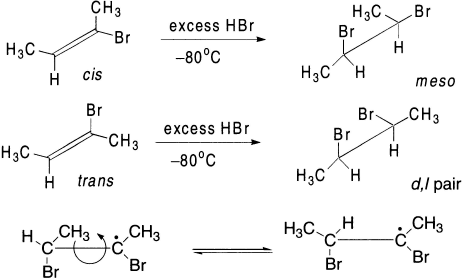
The third mechanism explains the formation of a bridged radical analogous to the bromonium ion. The HBr then attacks these radicals opposite to the bridge to yield stereospecific products.
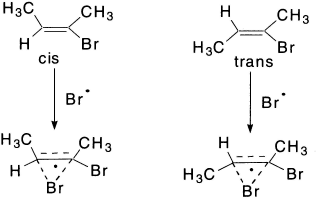
At higher temperatures and lower HBr concentrations these two radicals may interconvert to give nonstereospecific products.
A layer of ozone surrounding earth shields it from harmful solar radiation. The greater concentration of ozone occurs between 12 and 15 miles above earth’s surface in a part of the atmosphere called the stratosphere. Ozone is formed in the atmosphere by the reaction of short-wavelength ultraviolet light with molecular oxygen.
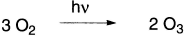
This stratospheric ozone layer acts as a filter for biologically harmful ultraviolet radiation. UV light can damage DNA in skin cells, causing mutations that trigger skin cancer. Since about 1985, scientists have noted a sharp drop in stratospheric ozone over Antarctica. This area of ozone depletion is known as the ‘ozone hole’.
Strong circumstantial evidence implicates synthetic chlorofluorocarbons (CFCs) as a major cause for ozone depletion. These gases, known commercially as Freons, have been extensively used as cooling fluids in refrigerators, in home and automobile air conditioners, in industrial cleaning solvents and in the manufacture of some plastic foams. They were once widely used as propellants in aerosol spray cans because of their odourless, nontoxic and nonflammable properties and because they are chemically inert.
Chlorofluorocarbons remain very stable in the atmosphere until they reach the stratosphere. There, they react with UV light that cause their homolytic cleavage, generating chlorine radicals. The chlorine radicals are the ozone-removing agents. They react with ozone to form chlorine monoxide free radicals and molecular oxygen. The chlorine monoxide radicals react with ozone to regenerate chlorine radicals. These two propagating steps are repeated over and over, destroying a molecule of ozone in each step. It has been calculated that each chlorine atom destroys 100,000 ozone molecules. Because of their remarkable chemical stability, CFCs have half-lives of 70–120 years.
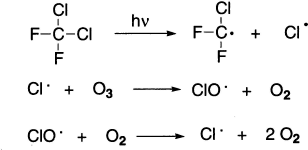
Although CFCs are to be phased out by international agreement, their concentration in the atmosphere continues to rise, and the concentration of ozone continues to drop. The hole in the ozone shield over the Antarctic continues to grow, and there have been indications that severe depletion is occurring over the Arctic as well.
Leave a Reply