The strengths of acids, as measured by pKa values, range from very weak ones such as hydrocarbons to acids that are much stronger than sulphuric acid. Acids with acidities equal to or greater than sulphuric acid are called super acids. Some of them are so acidic that they can donate a proton to an alkane, even to methane to form the CH5+ ion, first prepared by the American chemist George Olah. Such cations are said to be hypervalent because they formally possess ‘extra’ bonds, beyond those normally formed by second row atoms. Thus, they differ in character from the trivalent carbocations encountered as intermediates in electrophilic addition. There can be no ‘classical’ formalism for a hypervalent ion, because it does not conform to the rules of valency (C = 4, H = 1). In one possible ‘nonclassical’ structure, the added proton is associated side-on with the electron density of one of the C–H bonds of methane. These hypervalent ions are unstable and undergo loss of H2. Thus, the rates of electrophilic addition reactions increase with increasing stability of the intermediate cation. There are more than a hundred odd chemical elements, but only C, N, O and S form double bonds in the usual sense; and of these C and N form the true triple bonds, although nine different types of multiple bonds may be formed from the various combination of these elements.
In this lesson, we will consider the inverse reaction of the elimination that is the addition of an electrophile to one carbon atom of a multiple bond and a nucleophile to the other carbon atom. That is, we deal with additions of XY (where X and Y are single atoms or polyatomic groups) to double and triple carbon–carbon bonds, to give products in which X and Y are separately bonded to the adjacent carbons of the original multiple bond. The simplest are reactions of diatomic molecules HX or X2 where X is a halogen (most commonly chlorine or bromine), and these almost invariably have step-wise mechanisms. Alkenes decolourize bromine water, that is, they react with bromine to give colourless dibromoalkanes. Such additions to alkenes or alkynes are the reverse of elimination reactions.
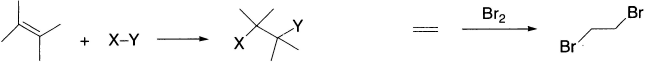
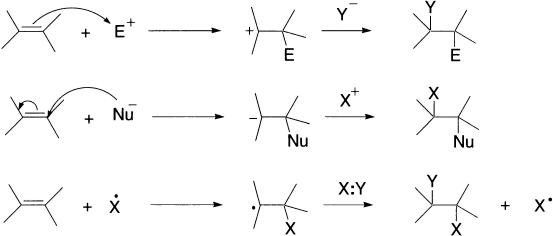
We shall discuss the mechanisms of addition in turn, according to their common names, that is, whether the initial step of the reaction of the unsaturated organic compound is with an electrophile, a nucleophile, or a free radical. In electrophilic addition, of course, the overall process is completed by the addition of a nucleophile; in nucleophilic addition, completion involves addition of an electrophile and in free radical addition the second step consists of a combination of the resultant intermediate with a neutral entity.
Leave a Reply