Here are two deceptively similar elimination reactions. The leaving group changes and the reaction conditions are very different, but the overall process is elimination of HX to produce one of two alkenes.
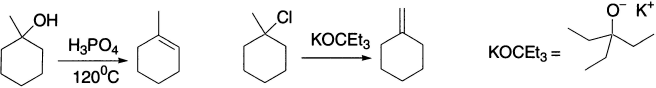
In the first example, acid-catalyzed elimination of water from a tertiary alcohol produces a trisubstituted alkene. Elimination of HCl from the corresponding tertiary alkyl chloride, promoted by a very hindered alkoxide base (more hindered than t-BuOK because all the ethyl groups have to point away from one another), gives exclusively the less stable disubstituted alkene.
The reason for the two different regioselectivities is a change in mechanism. As we have already discussed, acid-catalyzed elimination of water from tertiary alcohols is usually E1, and you already know the reason why the more substituted alkene forms faster in E1 reactions. It should come to you as no surprise now that the second elimination, with a strong, hindered base, is an E2 reaction. But why does E2 give the less substituted product? This time, there is no problem getting C–H bonds anti-periplanar to the leaving group: in the conformation with the Cl axial there are two equivalent ring hydrogens available for elimination, and removal of either of these would lead to the trisubstituted alkene. Additionally, any of the three equivalent methyl hydrogens is in a position to undergo E2 elimination to form the disubstituted alkene, whether the Cl is axial or equatorial–and yet it is these and only these that are removed by the hindered base. The diagram summarizes two of the possibilities.
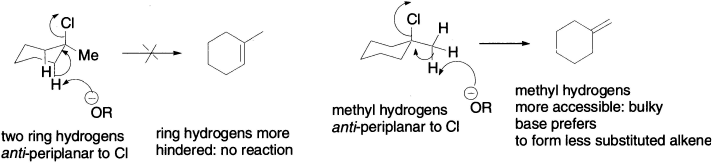
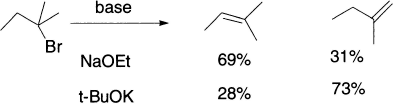
The base attacks the methyl hydrogens because they are less hindered–they are attached to a primary carbon atom, well away from the other axial hydrogens. E2 eliminations with hindered bases typically give the less substituted double bond, because the fastest E2 reaction involves deprotonation at the least substituted site. The hydrogens attached to a less substituted carbon atom are also more acidic. Think of the conjugate bases: a t-butyl anion is more basic (because the anion is destabilized by the three alkyl groups) than a methyl anion, so the corresponding alkane must be less acidic. Steric factors are evident in the following E2 reactions, where changing the base from ethoxide to t-butoxide alters the major product from the more substituted to the less substituted alkene.
Elimination regioselectivity
- E1 reactions give the more substituted alkene
- E2 reactions may give the more substituted alkene, but become more regioselective for the less substituted alkene with more hindered bases
Leave a Reply