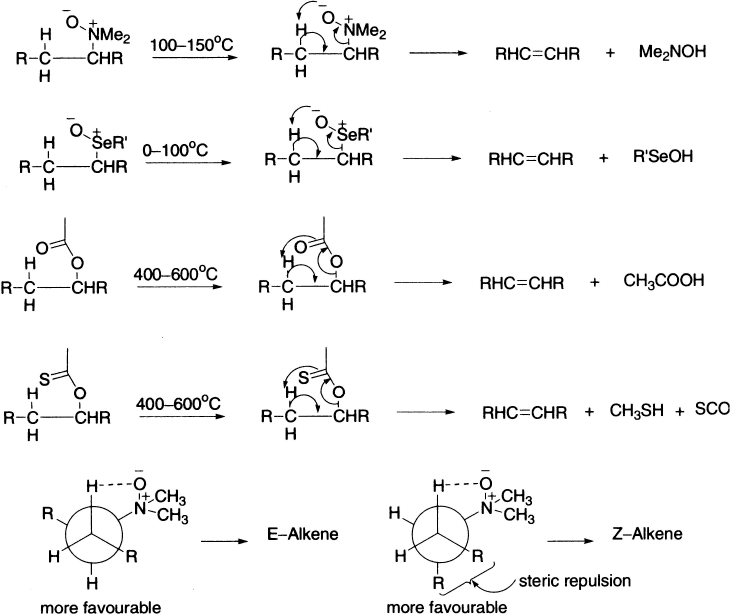
Some classes of compounds like acetates, xanthates and amine oxides undergo unimolecular reaction via a cyclic transition state, within which an intramolecular proton transfer is accompanied by elimination to form a new carbon–carbon double bond. These reactions are carried out in a gas phase under high temperature. The cyclic transition state exhibits syn-elimination, that is, the hydrogen atom and the leaving group depart from the same side of the incipient double bond. The mechanisms are obviously different from those already discussed, since all those require a base in one of the steps, and there is no base or solvent present in pyrolytic elimination. Two mechanisms have been found to operate: one of these is a cyclic concerted mechanism.
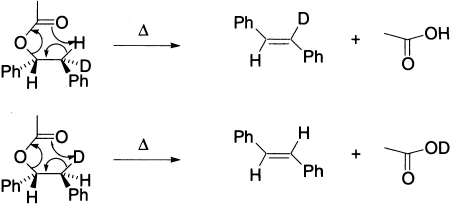
The above pyrolysis of erythro and threo isomers of 1-acetoxy-2-deutero-l,2-diphenylethane yielded, in each case, trans-stilbene, showing the existence of a cyclic, syn, concerted mechanism. As expected, there is complete retention of deuterium in the alkene obtained from the erythro isomer and its loss from the threo isomer. The molecule has two stereocentres, that is, four stereoisomers are possible: one pair of enantiomers in each erythro and threo isomer. In eclipsed conformations Ph/Ph θ = 0°. The elimination thus occurs from conformations with Ph/Ph far apart in the transition state.
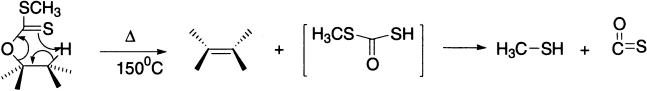
The synthetically important Tschugaev reaction involves pyrolysis of a xanthate at relatively low temperatures in a stereospecific manner.
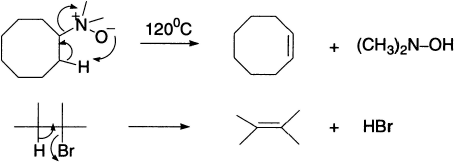
Pyrolysis of amine oxide yields alkene via a cyclic process. Alkyl halides also undergo pyrolytic dehydrohalogenation via a four-member cyclic transition state.
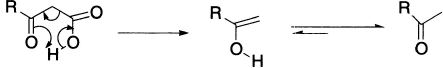
The ready decarboxylation of β-keto-acids has been shown to occur analogously,
If more than one type of β-hydrogens can attain the eclipsed conformation of the cyclic transition state, a mixture of alkenes will be formed. The product ratio parallels the relative stability of the competing transition states. Usually, more of the E-alkene is formed because of the additional eclipsed interactions present in the transition state leading to the Z-alkene.
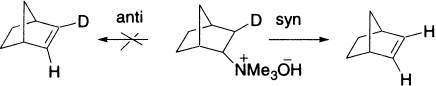
The rigid ring in N,N,N-trimethylnorbornyl ammonium ion prohibits attainment of an anti-elimination process.
The Cope reaction is a related pericyclic reaction of amine oxides, which involves a five-member cyclic transition state. It occurs under mild conditions and is useful in the generation of non-conjugated polyenes. For example:
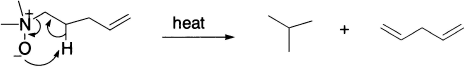
Since only one molecule of the substrate is involved in the reaction, the kinetics are of the first order. Free-radical inhibitors do not slow the reactions, so no free-radical mechanism is involved. The mechanism predicts exclusive syn-elimination. Some of these reactions have been shown to exhibit negative entropies of activation, indicating that the molecules are more restricted in geometry in the transition state than they are in the starting compound.
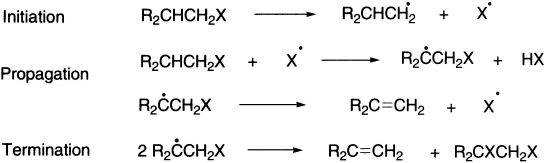
The second type of pyrolytic mechanism is free-radical mechanism. Initiation occurs by pyrolytic homolytic cleavage. Free-radical eliminations in solution are also known but are rare.
Leave a Reply