This effect involves the transfer of the electron-pair towards one of the atoms constituting a double bond in the presence of an attacking reagent. A familiar example is the reaction of cyanide ion with carbonyl compounds, which can be shown as:
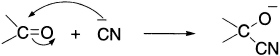
In this reaction, the electron-pair in the double bond is displaced towards the more electronegative oxygen atom (compared to carbon) as the reagent cyanide ion attacks the carbon of the C=O group. This effect is temporary and disappears when the attacking reagent is removed from the reaction site.
Leave a Reply