In simple terms, electrophilic aromatic substitution proceeds in two steps. Initially, the electrophile, E+, adds to a carbon atom of the benzene ring in the same manner as it reacts with an alkene, but here the π-electron cloud is disrupted, in the process giving the cationic species (arenium ion or substituted cyclohexadienyl cation). The cationic intermediate is, of course, less stable than the starting material, but as a cation it is reasonably stable because of delocalization around the six-member ring. However, in the second step, the resultant carbocation eliminates a proton to regenerate the aromatic system. This step is quite exothermic. The combined processes of addition and elimination result in overall substitution.
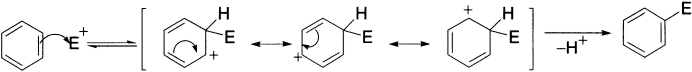
The reaction has been considered to proceed via the formation of a complex as an intermediate. Two types of such complexes have been discussed as probable intermediates. The first is σ-complex and the second is π-complex. The intermediate carbocation is stabilized by resonance, with the positive charge shared formally by three carbon atoms of the benzene ring. The resonance hybrid structure A indicates the delocalization of the charge. The carbocation is also referred to as a σ-complex or Wheland intermediate or benzenonium ion in which an electrophile is bonded tetrahedrally to a carbon atom of the aromatic ring.
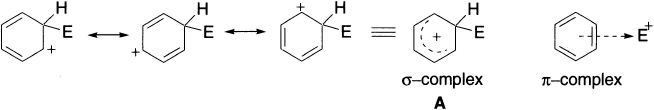
The hybridization state of the carbon atom that is attacked changes from sp2 to sp3 and the planar aromatic system is destroyed. Wheland complexes are high-energy intermediates devoid of conjugated aromatic electron sextet present in the product and in the starting material. Consequently, the formation of these complexes is the rate-determining step of the reactions. In the second step, a proton is abstracted by a basic species present in the reaction mixture. The attacked carbon atom reverts to sp2 hybridization, and planarity and aromaticity are restored. This fast step is energetically favourable and is regarded as the driving force for the overall process.
The π-complex involves a weak interaction between the π-electrons of the ring and the electrophile. In this case, there is no definite bond formation with the ring carbon atom and only the inductive effects of the substituents present on the aromatic ring stabilize the complex. These complexes have not been isolated but their existence has been proved from changes in the UV spectra. The well known silver ion-olefin adduct has been classified as a π-complex.

The energy changes that occur during the course of the reaction are related to the structural changes in the reaction profile shown in the following figure. It should be noted that each step proceeds through a high-energy transition state in which partial bonds attach the electrophile and the proton to the ring and the π-cloud is incomplete.
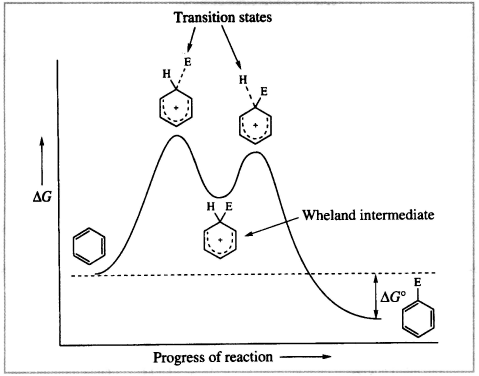
Figure 8.1 Energy profile for the electrophilic attack on benzene
Most examples of electrophilic aromatic substitution proceed by this sequence of events:
- Generation of an electrophile
- Attack of the aromatic ring on the electrophile
- The resulting carbocation is stabilized by resonance
- A proton is eliminated from the carbocation, regenerating the π-cloud
- A substituted aromatic compound is formed
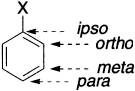
The product is a substituted benzene derivative. The position around the benzene ring relative to any substituent is named as follows:
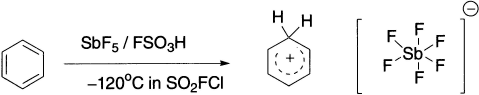
The cationic intermediate can be trapped using nonnucleophilic and nonbasic counter ion, such as SbF6−. In this octahedral anion, the fluorine atoms surround the central antimony atom and the negative charge is spread over all seven atoms. The protonation is carried out using FSO3H and SbF5 at −120°C.
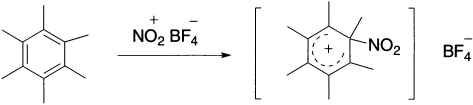
In some instances, the Wheland intermediate can be isolated. For example, when hexamethyl benzene is treated with the nitrating agent nitronium fluoroborate (NO2+ BF4−), the product cannot undergo proton loss and can be isolated at −70°C.
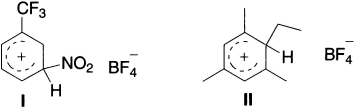
Olah and coworkers have shown that benzotrifluoride-nitryl fluoride-boron trifluoride system forms a yellow salt (I) that is stable upto −15°C and the mesitylene-ethylfluoride-boron trifluoride system form an orange salt (II), which melts at −15°C.
In the following sections, various examples are reviewed, highlighting the source of the electrophile and any variations in mechanistic detail.
Nitration of Benzene
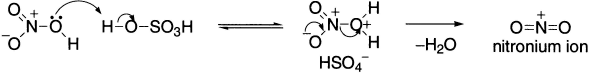
Introduction of nitro group into an aromatic system provides a general entry into aromatic nitrogen compounds. Among electrophilic substitutions, nitration is perhaps the most widely studied. Benzene cannot be nitrated using nitric acid alone, which lacks a strong electrophilic centre, but it is readily achieved using a mixture of concentrated nitric acid and concentrated sulphuric acid, the so-called ‘mixed acid’. The product is nitrobenzene. The interaction of nitric acid and sulphuric acid produces the electrophile, the nitronium ion (NO2+), which is linear with sp hybridized nitrogen at the centre. It is isoelectronic with CO2. The sulphuric acid is also the source of the base HSO4− that removes the proton in the second step.
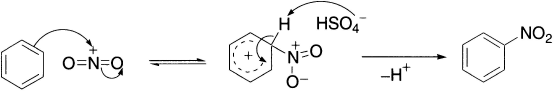
Other nitrating agents that have been mentioned in the literature are NO2+ BF4−, NO2+ClO4−, CH3COONO2, arylchloroformates and silver nitrate. Nitration is largely dependent on the reaction conditions and the nature of the substrate. Deactivated compounds such as nitrobenzene or benzoic acid exhibit second order kinetics, that is, the activated complex contains a molecule each of the aromatic and the NO2+ ion. The nitration of compounds that are less reactive than benzene such as ethyl benzoate or p–dichlorobenzene obeys a pseudo first order kinetics, the rate being dependent on the concentration of the substrate only. Compounds more reactive than benzene such as toluene or xylene show a zero order kinetics, that is, the rate is independent of the substrate.
Halogenation of Benzene
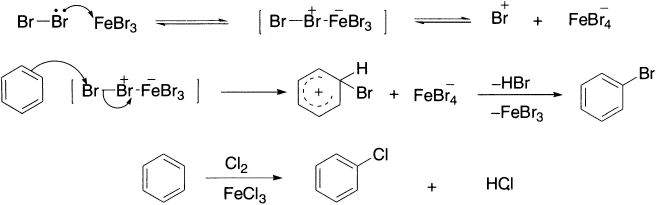
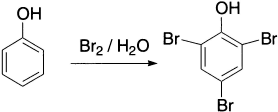
Halogen molecules (molecular Cl2 or Br2) are not strong electrophiles and do not react with benzene. However, in the presence of a Lewis acid, (a Lewis acid is a species that accepts an electron-pair and Lewis base donates an electron-pair) reaction occurs readily. The role of the catalyst is to accept a lone pair of electrons from the halogen molecule; this weakens the X-X bond (X = Cl or Br), which then becomes electron-deficient at one of the halogen atoms. The catalyst is referred to as halogen carrier. The actual electrophile is probably the complex formed from the halogen and the catalyst, rather than a halonium ion, for example, Cl+ or Br+. Bromination of benzene serves as a good example of halogenation. Hydrated Lewis acids are inactive as catalysts, so they are generally generated in situ to prevent hydration.
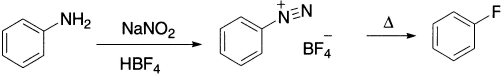
Fluorination with F2 itself is too vigorous to be of preparative value, as it results in a breakdown of the molecules being attacked. Aryl fluorides can be prepared by heating aryl diazonium tetrafluoroborates, which are prepared by the reaction of an arylamine with NaNO2 and fluoroboric acid, HBF4.
During the reaction of I2 and FeI3 with benzene, the equilibrium favours the starting materials and the aryl iodide is not obtained. Iodine itself is not reactive enough to attack benzene, even with the assistance of a catalyst, but will attack more reactive aromatic species. Direct iodination is difficult since iodine is the least active of the halogens. Electrophilic iodine (I+) is obtained by oxidizing I2 with an oxidizing agent such as nitric acid or mercuric oxide.
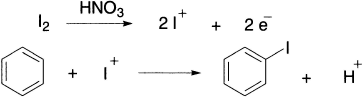
Friedel–Crafts Alkylation
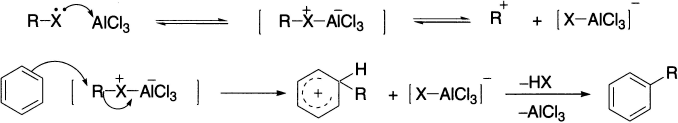
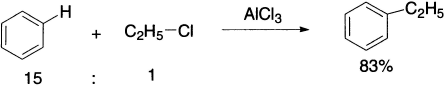
Charles Friedel (1832–1899), a French chemist, and James Crafts (1839–1917), an American mining engineer, both studied with Wurtz and then worked together in Paris, and in 1887 they discovered the Friedel–Crafts reaction. Alkyl halides require a Lewis acid catalyst to accentuate the polarization and create a more powerful electrophile. There is not enough positive character on the carbon atom in alkyl halides for them to react with benzene; the catalyst increases the positive character. Aluminium chloride is commonly used as the Lewis acid, accepting a pair of electrons from the halogen atom. Other commonly used catalysts are AlBr3, BF3, GaBr3 and TiCl4, etc. The electrophile may be a carbocation or perhaps more likely the complex shown. An alkyl benzene is produced.
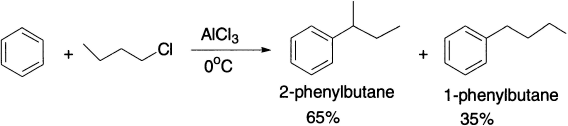
In the reaction of benzene with 1-chlorobutane, where rearrangement converts a primary carbocation into a secondary carbocation, 65% of the product is the rearranged product.
Of course, rearrangement of the alkyl group is not a problem if the alkyl group is not rearrangement prone, as in the following example.
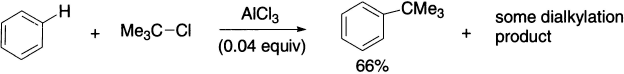
Another complication in Friedel-Crafts alkylation is that alkyl benzene products are more reactive than benzene itself. This means that the product itself can undergo alkylation, and a mixture of products is observed.

The monoalkylated product can be obtained in good yield, however, if a large excess of the starting material is used.
Friedel–Crafts Acylation
Acylation can be achieved using either acyl halides or acid anhydrides. The product is a ketone. Acyl halides are more reactive than the anhydrides, but still require a Lewis acid catalyst to promote the reaction. The attacking species is the resonance-stabilized acylium ion or the complex. Because the product of a Friedel–Crafts acylation reaction contains a carbonyl group that can complex with AlCl3, it must be carried out with more than one equivalent of AlCl3. When the reaction is over, water is added to the reaction mixture to liberate the product from the complex by hydrolyzing the aluminum salts. Acylation is better than alkylation. Indeed, alkylation is often carried out not directly, but via initial acylation followed by reduction of the acylated product. For example, ethyl benzene can be prepared by acylation of benzene with acetyl chloride followed by reduction.
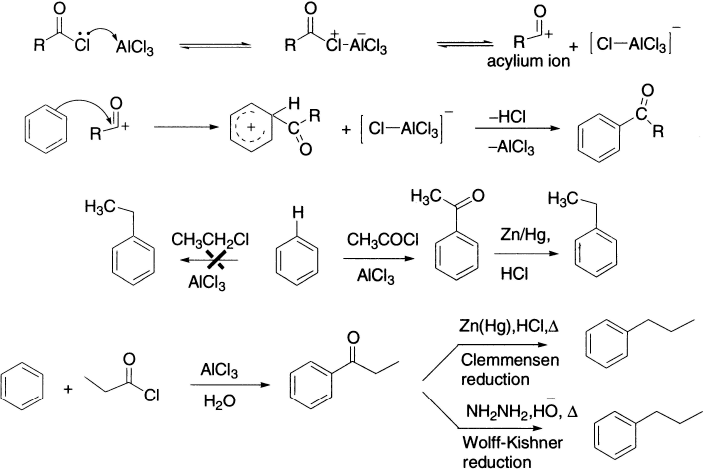
Solvents affect the distribution of products in the Fridel–Crafts acylation. For example, the acylation of anthracene in CS2 gives 9-acetylanthracene as the main product, whereas 1–acetylanthracene is obtained in nitrobenzene. The explanation may be given that the CH3COCl−AlCl3 complex solvolyzed by nitrobenzene is bulkier than in CS2; therefore, its attack at 1–position is favoured.
Sulphonation of Benzene
Benzene itself reacts very slowly or is not attacked by concentrated sulphuric acid, but is readily converted to benzenesulphonic acid by fuming sulphuric acid. This is a solution of 10–30% sulphur trioxide in concentrated sulphuric acid, and is known as oleum. Note here that the attacking electrophile is a neutral species and that the electron-deficient sulphur atom of SO3 is the electrophilic centre. Other reagents that form carbon–sulphur bonds are fluorosulphuric acid (FSO3H), chlorosulphuric acid (ClSO3H) and sulphurtrioxide (SO3). Sulphonation of benzene is a reversible reaction. If benzenesulphonic acid is heated in dilute acid, the reaction proceeds in a reverse direction.
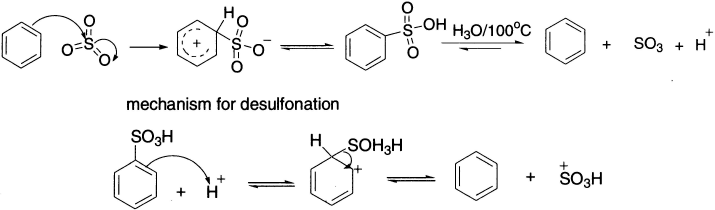
The distribution of isomers in the products of sulphonation depends on the temperature of the reaction and the concentration of the acid. Sulphonation of naphthalene forms 1-sulphonic acids between 0−40°C in 90% yields, but at 160°C the 2-isomer is obtained in about the same yield. Sulphonation differs from the other examples that have been discussed in that it can readily be reversed. Heating benzenesulphonic acid with dilute sulphuric acid or water converts it back to benzene.
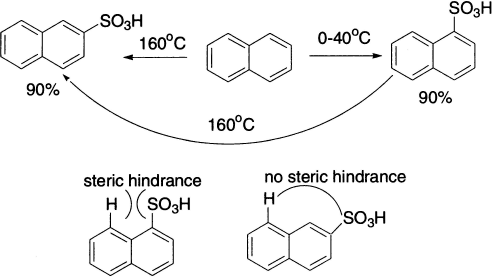
Because sulphonation is reversible, a sulphonic acid group is sometimes introduced to occupy a position on the ring, temporarily protecting that position from attack by another electrophile. Sulphonation is also a useful way of directing further substitution to a specific position.
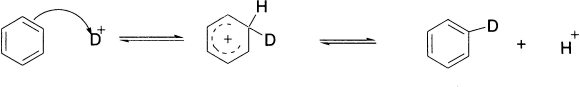
Protonation
Although benzene is a very weak base, it is protonated in concentrated sulphuric acid to a very slight extent. This reaction can be detected if the protonating mixture contains deuterium or tritium, the isotopes of hydrogen, since isotope exchange takes place. Some deuteriated benzene is produced when benzene is treated with D2SO4, and this can be detected by mass spectrometry and NMR spectroscopy. The more deuterium there is in the protonation mixture, the more exchange occurs. Notice that the regeneration of the aromatic system occurs by elimination of a proton.
Leave a Reply