Wolff rearrangement is an example of nucleophilic rearrangement involving carbene. It is a rearrangement of α-diazoketones leading to carboxylic acid derivatives via ketene intermediates, which is similar to the Curtius rearrangement. It can be achieved with metal catalysis or photochemically. There has been much debate about the timing of the mechanism and many attempts to distinguish between the two-step process involving the intermediacy of a carbene and a concerted process in which migration occurs at the same time as loss of nitrogen. The general consensus appears to be that carbenes are intermediates in the photochemically induced Wolff rearrangement, but there is still controversy about the thermal process.
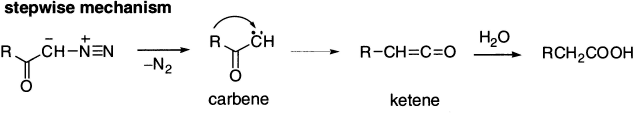
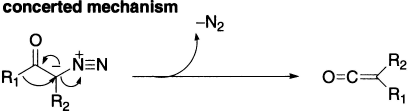
The above mechanism is supported by the following evidences: (a) the carbonyl carbon in the diazomethyl ketone becomes the carboxyl carbon in the resulting acid shown by 13C studies; (b) the migrating group R- migrates with retention of configuration and (c) in the absence of water or alcohol under favourable conditions, the intermediate ketene may be isolated.
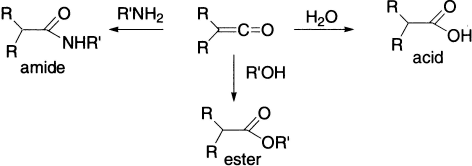
The actual product of the reaction is thus the ketene, which reacts with water, alcohols, or amines to give carboxylic acids, esters or amides, respectively.
Generally, the thermal reaction is carried out in the presence of silver salts, which catalyze the decomposition of the diazoketone. If the rearrangement is carried out in the presence of catalytic amount of silver (I) salts, the dediazotation of α-diazoketone A initially generates the ketocarbene B and/or the corresponding ketocarbenoid C, followed by 1,2-shift of the alkyl group to give the ketene D.
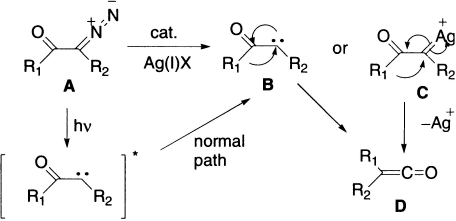
When the rearrangement is carried out photochemically, the same ketene D is formed. In this case, molecular nitrogen (N2) and an excited ketocarbene are formed initially, which may undergo relaxation to the normal ketocarbene B, which can then undergo the 1,2–shift to give the ketene D.
The diazoketone can exist in two conformations, called s-E and s-Z. Studies have shown that the Wolff rearrangement takes place preferentially from the s-Z conformation.

The reaction is of wide scope. R may be alkyl or aryl and may contain many functional groups including unsaturation, but not including groups acidic enough to react with CH2N2 or diazoketones.
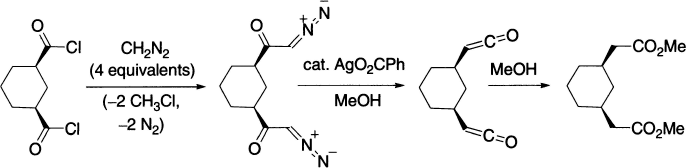
Two-fold Wolff rearrangement in the bis-homologation of dicarboxylic acids is reported. The substrate is a cis-disubstituted cyclohexane. The bisketene, which cannot be isolated, must have the same stereochemistry, because the dimethyl ester formed from the bisketene by the addition of methanol still is a cis-disubstituted cyclohexane.
The migrating group migrates with retention of configuration that has been confirmed by the following transformations involving optically active acid.
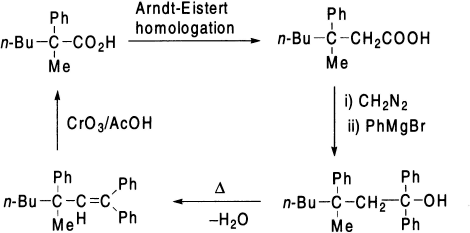
The acid obtained is degraded to the original acid by Barbier-Weiland degradation. The acid thus obtained is found to have the same configuration as the original acid.
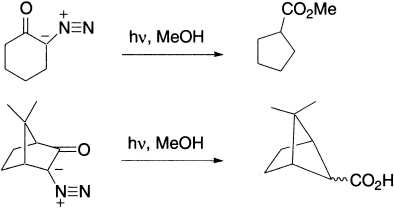
Wolff rearrangements are of particular utility in preparing ring-contracted carboxylic acids via cyclic α-diazoketones.
Leave a Reply