Beckmann Rearrangement
In the Beckmann rearrangement, an oxime is converted to an amide. An oxime is easily obtained by treatment of aldehyde or ketone with hydroxylamine. The OH group of ketoximes R1R2C(=NOH) can become a leaving group. Tosylation is one way to convert this hydroxyl group into a leaving group. The oxime OH group also can become a leaving group if it is either protonated or coordinated by a Lewis acid in an equilibrium reaction. Oximes activated in this fashion may undergo heterolysis. Because the formation of a nitrilium ion needs to be avoided, this heterolysis is accompanied by a simultaneous [1,2]–rearrangement of the group; the group that is attached to the C=N in the trans position with regard to the O atom of the leaving group. This is in complete contrast to the pinacol rearrangement where the nature of the rearrangement depends upon the relative migratory aptitudes of the migrating species. R1 and R2 may be alkyl, aryl, or hydrogen. However, hydrogen rarely migrates, so that the reaction is not generally a means of converting aldoximes to unsubstituted amides RCONH2. A nitrilium ion is formed initially. It reacts with water to form an imidocarboxylic acid, which tautomerizes immediately to an amide. The overall reaction sequence is called the Beckmann rearrangement. The Beckmann rearrangements of cyclic oximes result in lactams. The comparison of the structures of the starting ketone with those of the products reveals that the combination of oxime formation and Beckmann rearrangement accomplishes the insertion of an NH group between the carbonyl carbon and the α carbon.
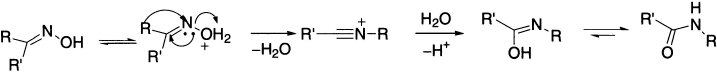
Beckmann rearrangement of the oxime of cyclohexanone is carried out on a very large scale industrially because the product, caprolactam, is the direct precursor of nylon 6, a versatile polymer that has many applications: for example, the manufacture of fibres for carpeting and other textiles. Concentrated sulphuric acid is used as both the acid catalyst and the solvent for the reaction.
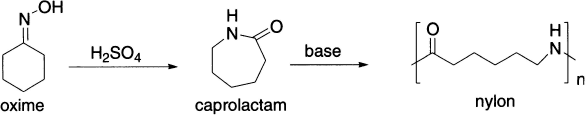
However, because caprolactam is soluble in sulphuric acid, the acid must be neutralized in order to isolate the product. Ammonia is used for this purpose and the large quantity of ammonium sulphate produced as by-product is sold as fertilizer.
The mechanism of the reaction follows the same pattern as a pinacol or Baeyer–Villiger reaction. Acid converts the oxime OH into a leaving group, and an alkyl group migrates on to nitrogen as water departs. The product cation is then trapped by water to give an amide.

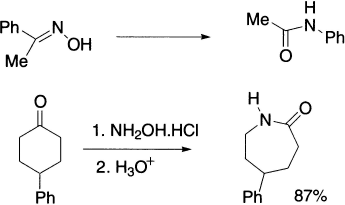
Stereospeciflcity in the Beckmann rearrangement is not as general as may be implied in some texts. However, the Beckmann rearrangement in general is stereospecific, and the group trans, that is, anti to the leaving group generally migrates. This method is often used to determine the configuration of the oxime. It has been observed that the migration actually assists ionization that is supported by rate and stereochemical evidences. For example, acetophenone oxime gives only acetanilide. If the migrating group is asymmetric, it retains its configuration during the reaction. Sometimes the group that was syn-to the hydroxyl is found to have migrated and, even more commonly, mixtures of amides may be formed—particularly if R1 and R2 are both alkyl groups.
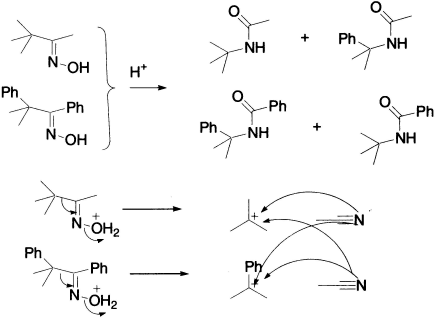
There are also two possible geometrical isomers of an unsymmetrical oxime. When mixtures of geometrical isomers of oximes are rearranged, mixtures of products result, which ratio mirrors exactly the ratio of the geometrical isomers in the starting materials.
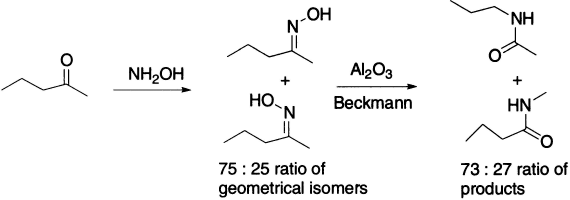
When a mixture of oximes with a tertiary centre next to oxime is subjected to a Beckmann rearrangement, crossover products are obtained showing fragmentation of the protonated oxime.
Oximes derived from aldehydes are not usually good substrates for the Beckmann rearrangement, and yields of primary amides obtained in this manner are commonly poor. However, cyclic ketones undergo a very useful ring enlargement to give cyclic lactams.
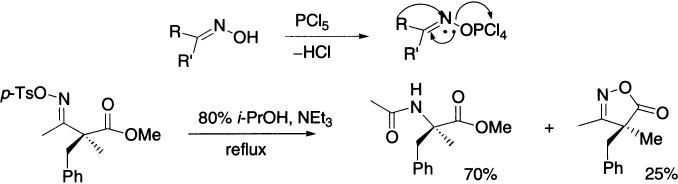
Toluene–p–sulphonyl chloride forms the oxime tosylate, which eliminates the stable tosylate anion. PCl5 and SOCl2 induce rearrangement by converting OH to a better leaving group.
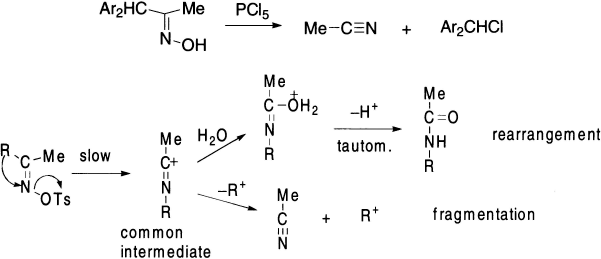
Certain ketoximes (oximes of α-diketones, α-keto acids, α-dialkylamino ketones, α-hydroxy ketones, ß-keto ethers) can be converted to nitriles by the action of proton or Lewis acids via fragmentation reactions, which are considered side reactions, often called ‘abnormal’ or ‘second-order’ Beckmann rearrangements.
Leave a Reply