When phenols are treated with acid chlorides or acid anhydrides, esters are obtained. Conversion of phenolic esters, on warming with a Lewis acid (Friedel-Crafts catalyst), to hydroxy ketones is known as Fries rearrangement
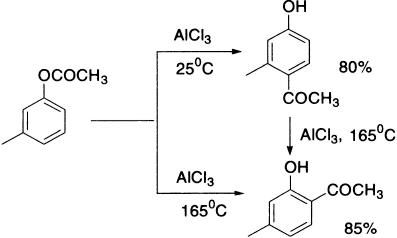
It is an acid-catalyzed rearrangement from side chains onto the aromatic ring. Acylation of phenols by Friedel-Crafts reaction also forms the same product mixture but the yield is poor. The relative amounts of ortho– and para–isomers in the product depends on the reaction conditions, that is, temperature, solvent and concentration of catalyst. The two isomers are separable and this is a useful method for the production of phenolic ketones. It has been observed that at low temperature the para–isomer predominates, whereas at higher temperature the more stable ortho is the major product. The para-product can be transformed into the ortho-product on further heating with AlCl3. Although the para–isomer is formed more rapidly, it appears that the greater stability of the ortho–isomer is due to the existence of intramolecular hydrogen bonding. In the presence of AlCl3, the complex formed is stabilized due to the union of two oxygen atoms through aluminium.

The exact mechanism of the Fries rearrangement is still uncertain and has been much disputed, with contradictory evidence being produced, as it is sometimes a one-step and sometimes a two-step process. It is believed that the initial step is probably the formation of a complex between the ester and the catalyst, followed by rearrangement leading to product. Evidence for the intermolecular process has been obtained from trapping experiments. Support for the intramolecular nature of the reaction is a formation of a π-complex intermediate between phenoxytrichloroaluminium and acylium ions. The mechanisms for one-step and two-step processes may be believed to proceed as follows:
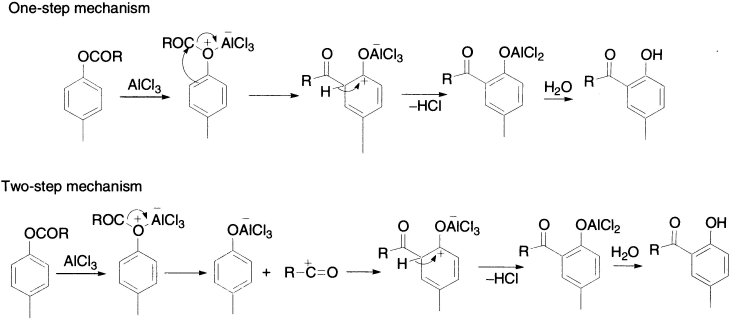
Evidence in support of the intermolecular mechanism is the formation of the crossover products.
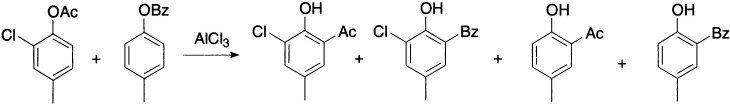
The Fries reaction can be carried out in the absence of a solvent, but the temperature at which reaction proceeds at a useful rate is lowered by the presence of solvents such as nitrobenzene and carbon disulphide. The usual catalysts are AlCl3, TiCl4, SnCl4 and BF3. The AlCl3 and phenyl ester are generally employed in approximately equimolar quantities.
The presence of a meta-directing group on the aromatic position of the phenyl ester usually interferes with the Fries reaction. For example, the reaction does not occur if the phenolic residue carries a nitro or benzoyl group in either the ortho– or meta-position; the presence of an acetyl or carboxyl group in the ortho-position hinders the reaction and in the para-position prevents it.
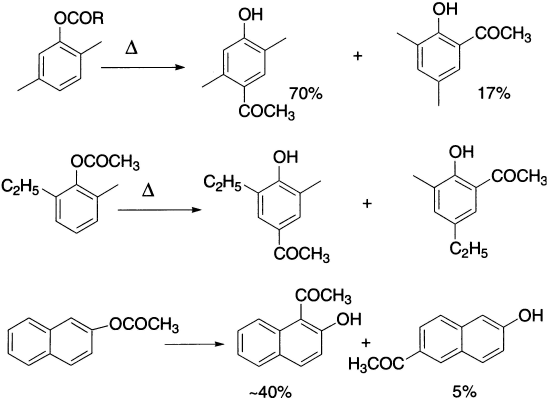
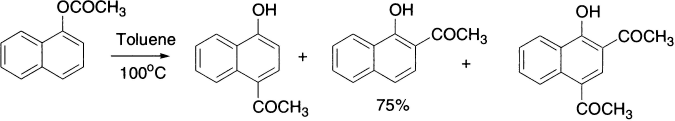
α-Naphthyl acetate undergoes Fries rearrangement at low temperature to give mainly 4-acetyl naphthol, but increasing the temperature increases the yields of 2-acetyl and 2,4-diacetyl naphthols.
The Fries rearrangement, carried out with UV light in the absence of a catalyst, and called photo-Fries rearrangement is predominantly an intramolecular free-radical process. Both ortho and para migrations are observed.
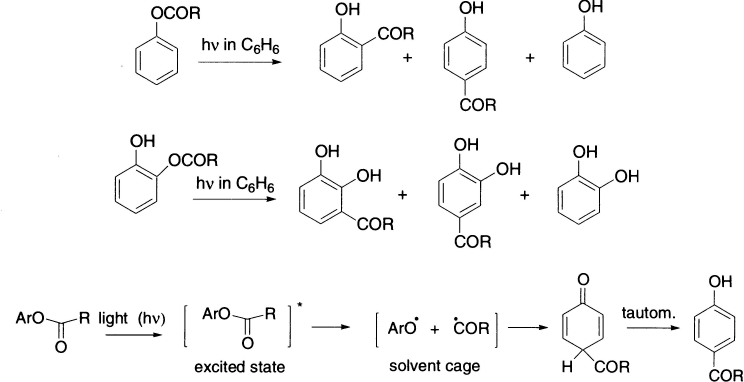
The phenol ArOH is always a side product, resulting from some ArO., which leaks from solvent cage and abstracts a hydrogen atom from a neighbouring molecule. When the reaction is performed on phenyl acetate in the gas phase, where there are no solvent molecules to form a cage, phenol is the chief product, and virtually no ortho-or para-hydroxy acetophenone is found. A photochemical variant of the Fries rearrangement is known; it has been shown spectroscopically to proceed via radical intermediates, the majority of which recombine before escaping their solvent cage.
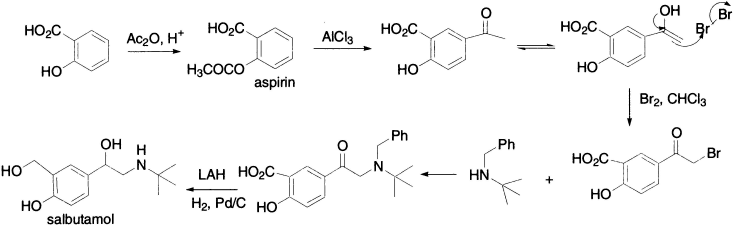
Salbutamol, an anti-asthma drug, is made from aspirin by Fries rearrangement. Halogenation of para–hydroxy ketone gives α–haloketone, which is an excellent electrophile and reacts rapidly with a nucleophile, such as a secondary amine, by the SN2 mechanism. Finally reduction of ketone and acid followed by removal of benzyl-protecting group gives salbutamol.
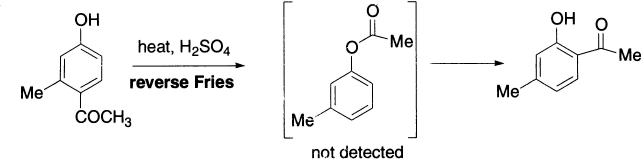
Some examples of a reverse Fries reaction have been found and this provides evidence to support the view that selective ortho-Fries rearrangement at high temperatures is a consequence of equilibrium via the esters to form the thermodynamically most stable 4-acylphenol. Indeed, 4-acylphenol may be converted to 2-acylphenol on heating with aluminium chloride, although the intermediate ester has never been detected under these conditions.
Leave a Reply