Transformation of a diketone to phenol in the presence of acid is known as the Dienone–Phenol rearrangement. As the name implies, this reaction results in the transformation of a quinoid structure to a benzenoid ring. It may be considered as reverse pinacol rearrangement, since pinacol and semipinacol rearrangements are driven by the formation of a carbonyl group. The dienone in acetic anhydride solution is treated with a catalytic amount of sulphuric acid at room temperature. The protonated carbonyl compound rearranges to a tertiary carbocation, which rapidly undergoes elimination of H+ to become aromatic. Thus, the driving force for the overall reaction is the creation of an aromatic ring in the product. In this rearrangement, the migration terminus is not the carbon of a protonated carbonyl group, but rather a carbon in conjugation with it.
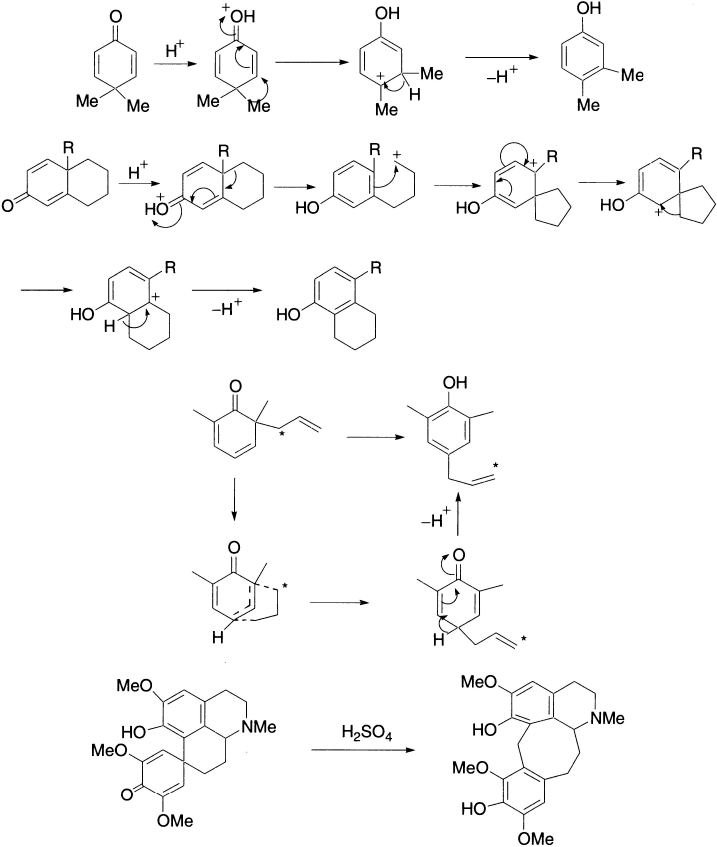
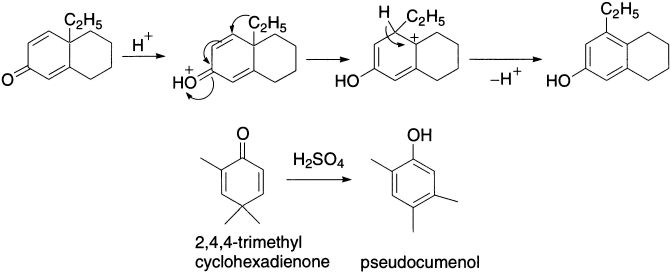
In the example just drawn, the ring methylene group migrates in preference to the primary alkyl group. The course of the reaction may be altered by slight structural or electronic changes in the molecule or by employing the aqueous condition. Thus, when R = C2H5, the ethyl group migrates, giving a different product. The group that can best support a positive charge usually prefers to migrate.
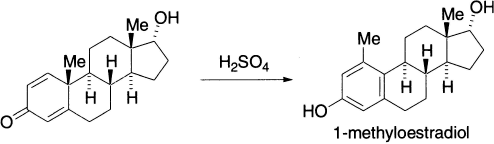
One of the important uses of dienone-phenol rearrangement is in the synthesis of a contraceptive pill, 1-methyloestradiol.
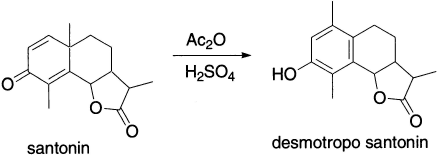
The rearrangement has strong connections with natural product chemistry.
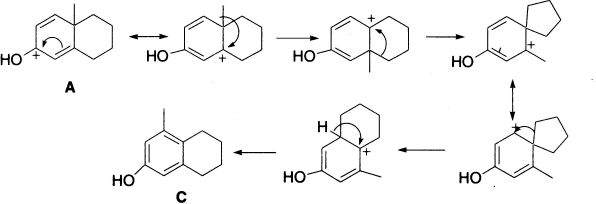
The preference for methylene over methyl migration is usually attributed to the greater ease of migration of a branched alkyl group over that of an unbranched alkyl group. Aryl groups migrate more readily than alkyl. Compound C could have been formed by the series of steps shown next.
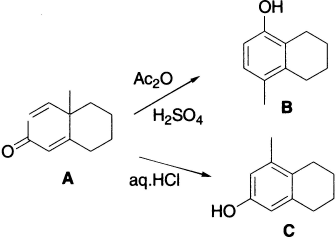
In aqueous acids the rearrangement places the hydroxyl group meta to the methyl group and in Ac2O solution, the rearrangement places the acetoxy group para to the methyl group.
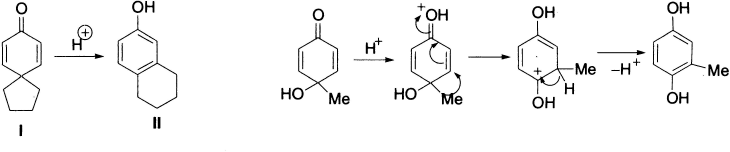
In a like manner, dienone I yields 6-hydroxytetralin II, and p-toluquinol when heated in aqueous methanol containing sulphuric acid rearranges to 2-methylquinol.
Leave a Reply