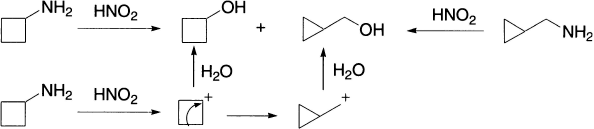
When a positive charge is formed on an alicyclic carbon, migration of an alkyl group may occur to give ring contraction by one carbon smaller than the original. This abnormal change involves conversion of more stable secondary carbenium ion to a less stable primary carbenium ion. Similarly, when a positive charge is formed on a carbon α to an alicyclic ring, ring expansion can take place. Both the old and new carbenium ions may give products by combination with a nucleophile, or by elimination. For example, cyclobutylmethylamine and cyclopropylmethylamine give similar mixtures of the two alcohols on treatment with nitrous acid.
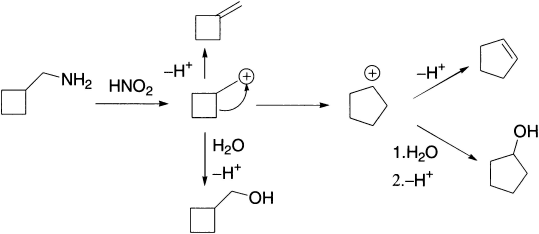
When the carbenium ion is formed by diazotization of the amine, the reaction is called the Demjanov rearrangement. The formation of cyclopropylmethyl cation may be due to its relative stability, by significant charge delocalization involving the ring bonds and the positive charge, since the C-C bonds in cyclopropane have appreciable p character.
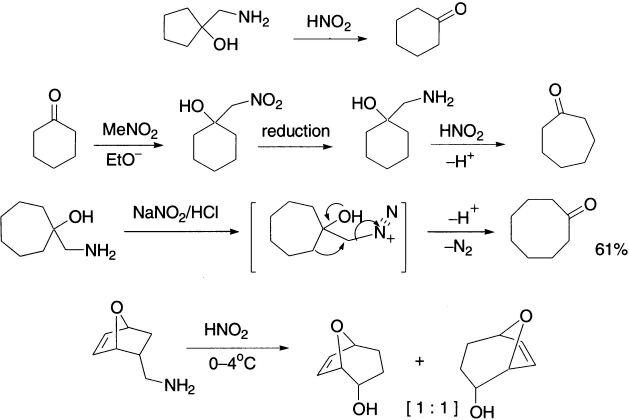
The expansion reaction has been carried out on rings of the size C3 to C8, but the yields are better with the smaller rings, because relief of small–angle strain provides a driving force for the reaction. The contraction reaction has been applied to four-member rings and to rings of C6 to C8. Treatment of 1–aminomethyl cyclopentanol with nitrous acid gives cyclohexanone in good yields. This reaction is called the Tiffeneau–Demjanov ring expansion. This reaction is of general use for the ring expansion of cycloalkanones to their next higher homologs. Some other examples of cyclic ß–aminoalcohols that undergo Tiffeneau–Demjanov rearrangement are as follows.
Aliphatic diazonium salts are much less stable than aromatic diazonium salts. The first reason for this difference is that aliphatic diazonium salts lack stabilization through resonance. Second, aliphatic diazonium salts release N2 much more readily than their aromatic analogs, as they thus react to give relatively stable alkyl cations. Hence, the decomposition of aliphatic diazonium ion is favoured by product–development control. The molecular nitrogen in aliphatic diazonium ions is an excellent leaving group.
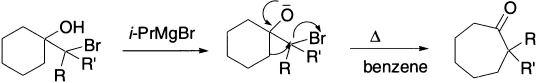
Cyclic bromohydrin on treatment with Grignard reagent gives alkoxide, which upon refluxing undergoes ring expansion. This reaction has been performed with bromohydrins in which the carbon-bearing bromine at least has one phenyl or methyl group, but not when both R and R′ are hydrogen.
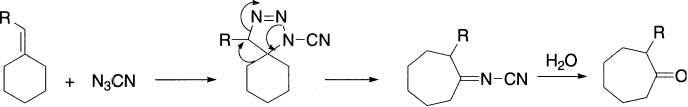
A related ring expansion involves treatment of an exocyclic olefin with cyanogen azide. The azide adds to the double bond in a 1,3-dipolar addition to give a triazolidine intermediate which undergoes ring expansion.
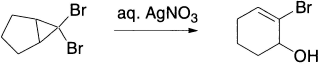
Cyclopropyl halides and tosylates give allylic products via allylic cation.
Leave a Reply