The shift of a hydrogen atom from one carbon atom in a carbocation to a neighbouring carbon atom is often quite rapid when a more stable carbocation can be formed from a less stable one. For example, when 2-methyl-1-propanol is treated with aqueous acid, water is lost and a tertiary carbocation is formed, as hydrogen shifts from C-2 to C-1, taking with it the electrons of the C-H σ bond.
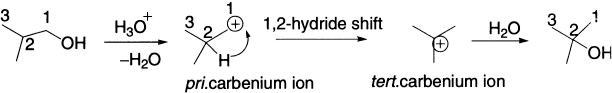
The addition of water to the resulting tertiary cation results in the formation of 2-methyl-2-propanol. Because the position of the hydroxyl group in the product alcohol has changed from its original position in starting alcohol, this reaction is classified as an isomerization rather than a rearrangement; the order in which atoms are joined has changed but the carbon skeleton is unchanged. In other words, the identity of the atom to which the functional group is bonded has changed, but the sequence of attachment of carbon atoms along the backbone is the same. However, the skeleton would have been altered if a carbon atom with its substituents, rather than hydrogen, had migrated to the developing carbocationic centre.
Leave a Reply