The Baeyer–Villiger oxidation (discovered in 1899 by the Germans Adolf van Baeyer, who won the Nobel Prize in 1905, and V. Villiger) involves the reaction of a ketone with H2O2 or a peroxy acid (RCO3H) to give an ester. Here, the starting material is a ketone, in which an oxygen atom is inserted between the carbonyl group and the α carbon to form an ester, that is, it involves 1,2-migration from carbon to electron-deficient oxygen.
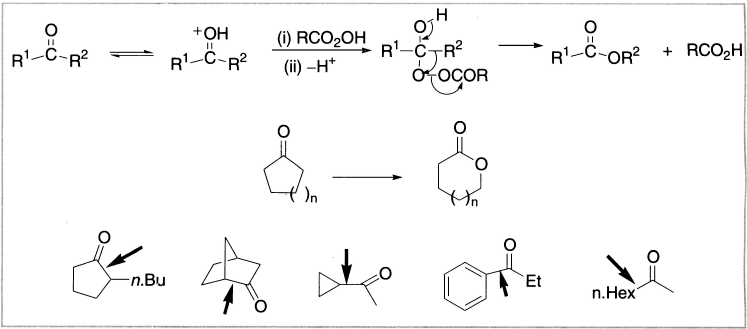
Figure 7.1 Sites of oxidation of some representative ketones
The Baeyer–Villiger oxidation begins with nucleophilic attack of the peracid on the ketone carbonyl. It takes place when a ketone is treated with a peracid, a carboxylic acid that has one additional oxygen. Peracids are powerful oxidizing agents, and this reaction is called an oxidation even though, as we will see, it is quite similar mechanistically to the rearrangement already discussed. Carboxylates are not good leaving groups, but the O-O single bond is very weak, and monovalent oxygen cannot bear to carry a positive charge so that once the peracid has added, loss of carboxylate is concerned with a rearrangement, driven, as in the case of pinacol, by formation of a carbonyl group.
In certain instances, information about the movement of atoms between molecules during the course of a reaction can be gained by using compounds containing isotopes of certain of the atoms. These isotopes behave much like the ordinary atoms they replace, but they can be identified by their behaviour. For example, in the hydrolysis of ethyl acetate, it is crucial to a determination of the mechanism to be able to establish which of the two reactants (ethyl acetate or water) provides the oxygen atom that ends up in the product ethyl alcohol. In this case, the use of water labelled with 18O reveals that the oxygen atom in the alcohol comes from the ethyl acetate molecule.
One important piece of evidence for this mechanism is that 18O labelled benzophenone gave ester entirely labelled in the carbonyl oxygen, with none in the alkoxyl oxygen. Carbon-14 isotope–effect studies on acetophenones have shown that migration of aryl groups takes place in the rate-determining step, demonstrating that migration of Ar is simultaneous with the departure of OCOR.
The most common peracids employed for Baeyer–Villiger oxidation are m-chloroperbenzoic acid (MCPBA) and peracetic acid as they are commercially available. MCPBA is crystalline and relatively stable when pure. However, it is somewhat more expensive than peracetic acid, which can be prepared in solution simply by adding a catalytic amount of sulphuric acid to a mixture of acetic acid and hydrogen peroxide. All peracids are very unstable in the presence of metals and metal ions. Even atmospheric dust contains a sufficient concentration of metal ions (such as iron oxides) to catalyze the decomposition of a peracid to form the acid and molecular oxygen.
Other reagents, which have been used to accomplish the same conversion, include H2O2-BF3.Et2O and K2S2O8-H2SO4. One limitation of this reaction is that double bonds present in the molecule are frequently epoxidized, and thioethers are oxidized to sulphoxides or sulphones.
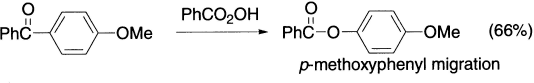
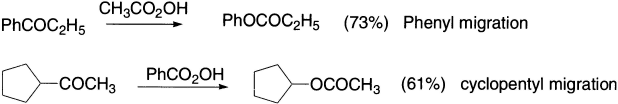
It should be noted that in the Baeyer–Villiger oxidation, if there is competition between two migrating groups, that group migrates which is more nucleophilic of the two. If the migrating group can take some responsibility for the positive charge, the transition state will be more stable. The more stable the charge, the faster the rearrangement. The migrating ability of the aryl groups is increased by electron-donating and decreased by electron-withdrawing groups.
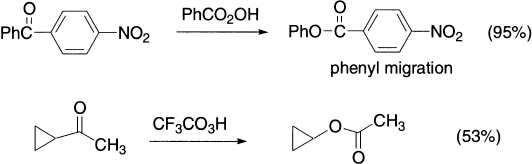
Enolizable ß-diketones do not react and α-diketones can be converted to anhydrides. With aldehydes, migration of hydrogen gives the carboxylic acid; migration of other groups would give formates.
As in the Baeyer–Villiger oxidation, the transition state is cationic, so the cation-stabilizing group migrates more readily. When a phenyl ring migrates, π participation is involved, as the benzene ring acts as a nucleophile and the positive charge can be spread out even further.
Aryl groups migrate more readily than alkyls, and a secondary alkyl more readily than a primary alkyl.
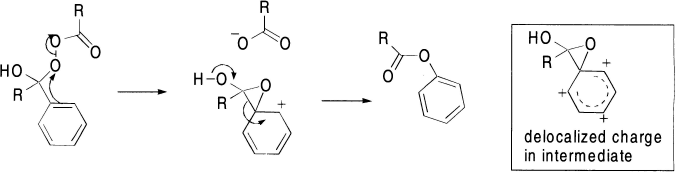
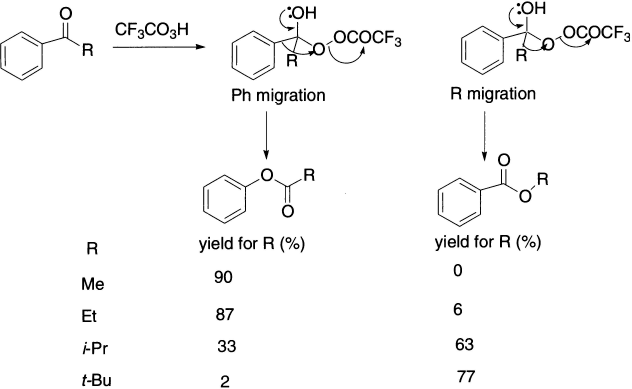
This acid-catalyzed reaction is similar to the formation of a hemiketal or a ketal from a ketone and an alcohol. Protonation of the carbonyl group activates it toward nucleophilic attack by the terminal oxygen of the peracid. Then, via a cyclic transition state, the carbon–oxygen π bond is reformed, with loss of a molecule of carboxylic acid, as the alkyl group migrates to oxygen.
This step is similar to a Beckmann or Hofmann rearrangement, except that the leaving group is a carboxylic acid and the heteroatom to which the group migrates is oxygen. The products of this unimolecular rearrangement are the ester derived from the ketone and the acid derived from the peracid. In 1,2-migrations, the migrating group retains its stereochemistry.
The order, with t-alkyl the best at migrating, then s-alkyl closely followed by Ph, then Et, then Me, very roughly follows the order in which the groups are able to stabilize a positive charge.
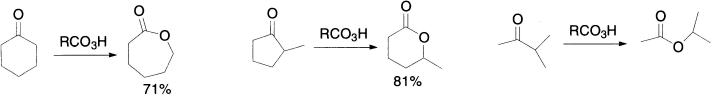
Baeyer–Villiger oxidation can be used with either acyclic or cyclic ketones. For example, the Baeyer–Villiger oxidation of cyclohexanone generates a lactone (a cyclic ester). With unsymmetrical ketones, the more substituted carbon migrates preferentially, as in the Beckmann rearrangement.
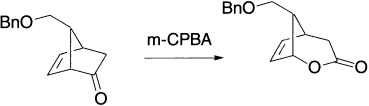
Unsaturated ketones are not often good substrates for Baeyer–Villiger oxidation. The two factors that matter are: how electrophilic is the ketone and how nucleophilic is the alkene. The following unsaturated bicyclic ketone undergoes Baeyer–Villiger oxidation.
The rate of oxidation is accelerated by electron-donating groups in the ketone and by electron-withdrawing groups in the peracid. The reaction occurs with retention of configuration. As in other 1,2-cationic rearrangements, the migrating group retains its stereochemical configuration. Some of the examples of carbon to electron-deficient oxygen migrations are as follows:
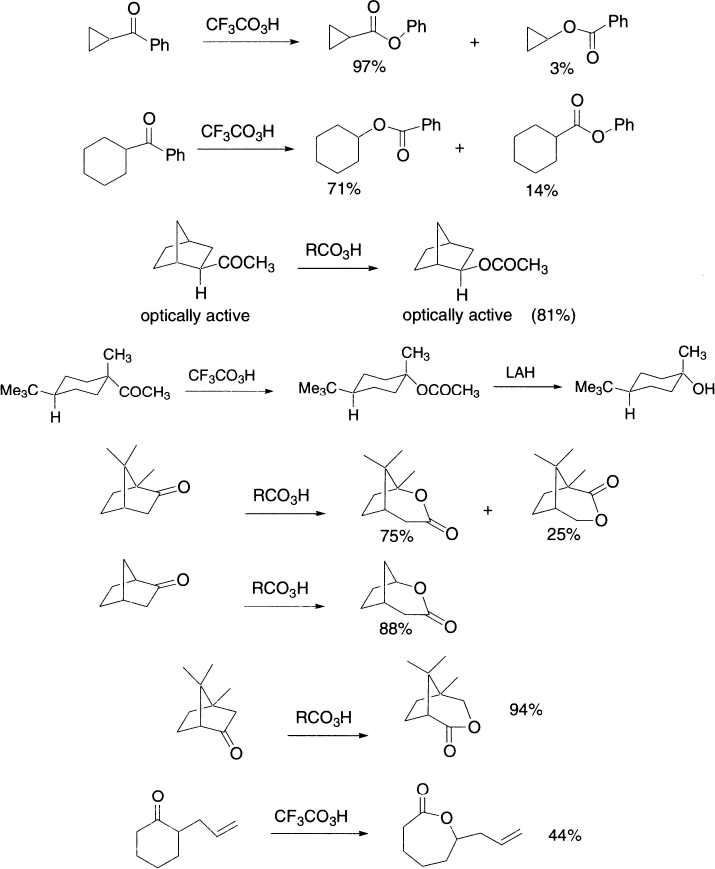
In the rearrangement of trans-9-decalylperbenzoate, the benzoate ion remains intimately associated with the cation at all times. Externally added anions fail to compete and interestingly, the two oxygens of the benzoate ion do not even equilibrate.
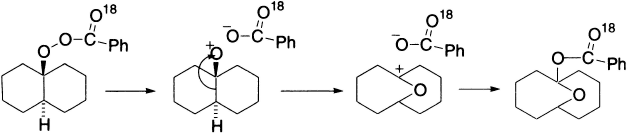
Here is an example of regioselective Baeyer–Villiger rearrangement of an electron-rich aromatic aldehyde.
Leave a Reply