Certain structural units containing heteroatoms can be substituted into conjugated systems in such a way that the system remains conjugated and isoelectronic with the original hydrocarbon. The most common examples are -CH=N- and -N=N- double bonds and divalent sp2 -O-, -S- and -NR- units. Each of these structural fragments can replace a -CH=CH- unit in a conjugated system and contribute two π electrons. These compounds are called heteroaromatic to recognize both the heterocyclic structure and the relationship to benzene and other aromatic structures.
MO calculations on compounds in which a -CH=N- unit replaces -CH=CH indicate that the resonance stabilization is very similar to that of the original compound. For the -O-, -S- and -NR- fragments, the resonance stabilization is somewhat reduced but, nevertheless, high enough to consider the resulting compounds to be aromatic in character. Various approaches have been used to estimate the aromaticity of these compounds. Generally speaking, the various approaches suggest that the aromatic stabilization of pyridine is similar to that of benzene. This is in agreement with the non-chemical estimates of the pyridine stabilization energy. Typically, the five-member compounds are found to be somewhat less stabilized than benzene, with resonance energies in the range of one-half to three-quarters of that for benzene. Magnetic and polarizability criteria put the order of aromaticity as thiophene > pyrrole > furan.
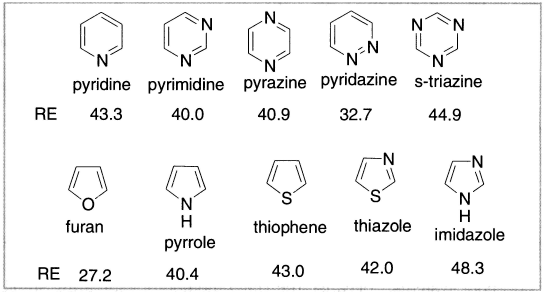
Figure 12.5 Structures isoelectronic with benzene
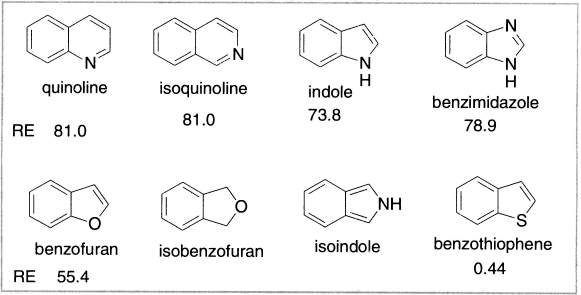
Figure 12.6 Structures isoelectronic with naphthalene
Additional heteroaromatic structures can be built up by fusing benzene rings to the aromatic heterocyclic rings or by fusing together heterocyclic rings. When benzene rings are fused to the heterocyclic five-member rings, the structures from fusion at the 2,3-positions are much more stable than those from fusion at the 3,4-positions. The π-electron system in the 3,4-fused compounds is more similar to a peripheral 10-π-electron system than to the 10-electron system of naphthalene. As a result, these compounds have a strong tendency to undergo reactions that restore benzene conjugation in the carbocyclic ring. The isobenzofuran structure is known to be an exceptionally reactive diene.
Leave a Reply