Many completely conjugated hydrocarbons can be built up from the annulenes and related structural fragments. It is of interest to be able to predict the stability of such fused-ring compounds. Because Huckel’s rule applies only to monocyclic systems, it cannot be applied to the fused-ring compounds, and there have been many efforts to develop relationships that would predict their stability. The underlying concepts are the same as for monocyclic systems. Stabilization should result from a particularly stable arrangement of molecular orbitals, whereas instability would be associated with unpaired electrons or electrons in high-energy orbitals.
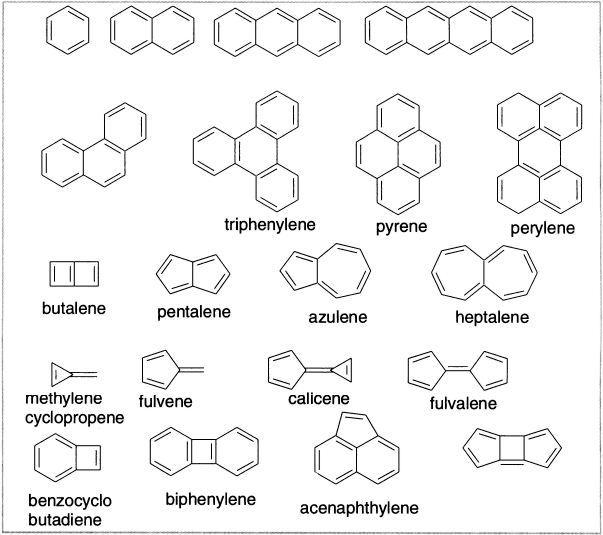
Figure 12.4 Some representative examples of polyenes
However, attempts to correlate stability with the Huckel delocalization energy, relative to isolated double bonds, give poor correlation with the observed chemical properties of the compounds. By choosing a polyene as the reference state, much better agreement between calculated stabilization energy and experimental chemical properties is achieved. Hess and Schaad have empirically established a series of energy terms corresponding to the structural units in the reference polyene. The difference between the energy of the conjugated hydrocarbon by HMO calculation and the sum of the energies of the appropriate structural units gives stabilization energy. For azulene, for example, the HMO calculation gives energy of 10α+13.36β. The energy for the polyene reference is obtained by summing contributions for the component bond types: 3(HC=CH) + 2(HC=C) + 3(HC–CH) + 2(HC–C) + 1(C–C) = 10α+13.13β. The difference, 0.23β, is the stabilization or resonance energy assigned to azulene by this method. For comparison of non-isomeric molecules, the Hess-Schaad treatment uses resonance energy per electron, which is obtained simply by dividing the calculated stabilization energy by the number of π electrons. Although the resulting stabilization energies are based on a rudimentary HMO calculation, they are in good qualitative agreement with observed chemical stability.
All these approaches agree that benzene and the structures that can be built up by fusing together benzenoid rings are strongly stabilized relative to the reference polyenes. The structures with more rings tend to have lower resonance energies per π electron compared to benzene. This feature is in agreement with experimental trends in reactivity. Because the structures with fewer rings are more stable, there is a tendency for species in which several rings are fused together to react by addition to an internal ring to give the smaller and more stable structures.
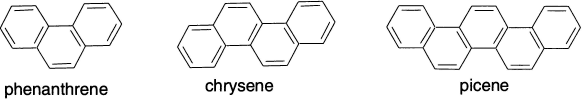
This trend is revealed, for example, by the rates of Diels-Alder addition reactions of anthracene, naphthacene and pentacene, in which three, four and five rings, respectively, are linearly fused. Benzene rings can also be fused in angular fashion, as in phenanthrene, chrysene and picene. These compounds, while reactive toward additions in the centre ring, retain most of the resonance energy per electron (REPE) stabilization of benzene, naphthalene and phenanthrene.
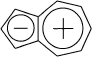
Azulene is one of the nonbenzenoid hydrocarbons that appear to have appreciable aromatic stabilization. Naphthalene is more stable than azulene by about 38.5 kcal/mol. The structure of azulene has been determined by both X-ray crystallography and electron-diffraction measurements. The bond shared by two rings is significantly longer, indicating that it has a predominantly single-bond character. The molecule has C2v symmetry indicating delocalization of π electrons. The molecule has dipolar structure as the fusion of a cyclopentadienide anion and cycloheptatrienyl cation. It has appreciable dipole moment (0.8D).
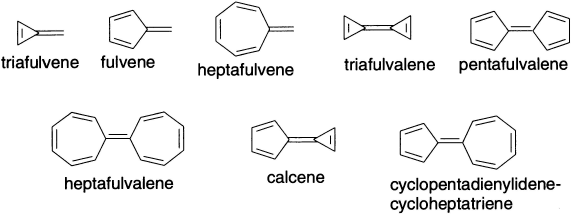
The possibility of extra stabilization in conjugated systems that have conjugated components exocyclic to the ring has also been examined. Some representative examples are as below:
Leave a Reply