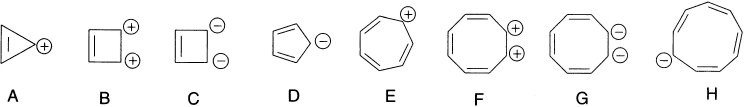
There are also striking stability relationships due to aromaticity in charged ring systems. These energy levels are applicable to charged species as well as to the neutral annulenes. A number of cations and anions that have completely conjugated planar structures are shown below. The Huckel rule predicts aromatic stability for cyclopropenium ion (A), cyclobutenium dication (B), cyclobutadiene dianion (C), cyclopentadienide anion (D), cycloheptatrienyl cation (tropylium ion, E), the dications and dianions derived from cyclooctatetraene (F and G) and the cyclononatetraenide anion (H). The other species having 4nπ electrons would be expected to be very unstable.
There is a good deal of information about the cyclopropenium ion that supports the idea that it is exceptionally stable. The cyclopropenium ion and a number of derivatives have been generated by ionization procedures:
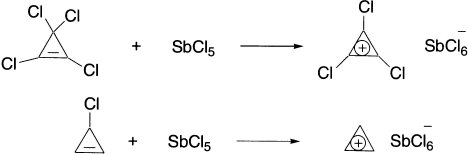
The 1,2,3-tri-t-butylcyclopropenium cation is so stable that the perchlorate salt can be recrystallized from water. An X-ray study of triphenylcyclopropenium perchlorate has verified the existence of the carbocation as a discrete species. The heterolytic gas-phase bond dissociation energy to form cyclopropenium ion from cyclopropene is 225 kcal/mol. This compares with 256 kcal/mol for formation of the allyl cation from propene or 268 kcal/mol for formation of the 1-propyl cation from propane.
In contrast, the less strained four-π-electron cyclopentadienyl cation is very unstable. It is calculated to have negative stabilization energy of 56.7 kcal/mol. The cyclopentadienyl cation is also found to be anti-aromatic on the basis of magnetic susceptibility and chemical shift criteria. The heterolytic bond dissociation energy to form the cation from cyclopentadiene is 258 kcal/mol, which is substantially more than for formation of an allylic cation from cyclopentene, but only slightly more than the 252 kcal/mol for formation of an unstabilized secondary carbocation. Solvolysis of cyclopentadienyl halides assisted by silver ion is extremely slow, even though the cyclopentadienyl ring is doubly allylic. When the bromide and antimony pentafluoride react at −78°C, an EPR spectrum is observed which indicates that the cyclopentadienyl cation is a triplet, but the ground state of the pentaphenyl derivative is a singlet.
The relative stability of the anions derived from cyclopropene and cyclopentadiene by deprotonation is just the reverse of the situation for the cations. Cyclopentadiene is one of the most acidic hydrocarbons known, with a pKa of 16.0. The pKs of triphenylcyclopropene and trimethylcyclopropene have been estimated as 50 and 62, respectively, from electrochemical cycles. The unsubstituted compound would be expected to fall somewhere in between and thus must be about 40 powers of 10 less acidic than cyclopentadiene. Thus, the six-π-electron cyclopentadienide ion is enormously stabilized relative to the four-π-electron cyclopropenide ion, in agreement with the Huckel rule.
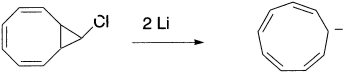
The Huckel rule predicts aromaticity for the six-π-electron cation derived from cycloheptatriene by hydride abstraction, and anti-aromaticity for the planar eight-π-electron anion that would be formed by deprotonation. The cation is indeed very stable. Salts containing the cation can be isolated as a product of a variety of preparative procedures. On the other hand, the pKa of cycloheptatriene has been estimated at 36. This value is similar to those of normal 1,4-dienes and does not indicate strong destabilization. Thus, the seven-member eight-π-electron anion is probably nonplanar. This would be similar to the situation in the nonplanar eight-π-electron hydrocarbon, cyclooctatetraene. The cyclononatetraenide anion is generated by treatment of the halide with lithium metal. The NMR spectrum of the anion, however, is indicative of aromatic character.
Several doubly charged ions have been observed experimentally. Ionization of 3,4-dichioro-l,2,3,4-tetramethylcyclobutene in SbF5-SO2 at −75°C results in an NMR spectrum attributed to the tetramethylderivative of the cyclobutadienyl dication. It is difficult to choose a reference compound against which to judge the stability of the dication. That it can be formed at all, however, suggests special stabilization associated with the two-π-electron system. The dianion formed by adding two electrons to the π system of cyclobutadiene also meets the 4n + 2 criterion. In this case, however, four of the six electrons would occupy nonbonding orbitals, so high reactivity could be expected. There is some evidence that this species may have a finite existence. Reaction of 3,4-dichlorocyclobutene with sodium naphthalenide, followed a few minutes later by addition of methanol-d6, gives a low yield of 3,4-di-deuterio-cyclobutene. The inference is that the dianion [C4H42−] is present. As yet, however, no direct experimental observation of this species has been accomplished. Cyclooctatetraene is reduced by alkali metals to a dianion.
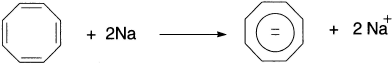
The NMR spectrum indicates a planar aromatic structure. It has been demonstrated that the dianion is more stable than the radical anion formed by one-electron reduction, as the radical anion is disproportionate to cyclooctatetraene and the dianion.
The crystal structure of the potassium salt of 1,3,5,7-tetramethyl-cyclootatetraene dianion has been determined by X-ray diffraction. The eight-member ring is planar, with ‘aromatic’ C–C bond lengths of about 1.41 A without significant alternation. The spectroscopic and structural studies lead to the conclusion that the cyclooctatetraene dianion is a stabilized delocalized structure. A dication derived from 1,3,5,7-tetramethyl-cyclooctatetraene is formed at −78°C in SO2C1 by reaction with SbF5. Both the proton and carbon NMR spectra indicate that the ion is a symmetrical, diamagnetic species, and the chemical shifts are consistent with an aromatic ring current. At about −20°C, this dication undergoes a chemical transformation to a more stable dication.
Reduction of the non-aromatic polyene [12]-annulene, either electrochemically or with lithium metal, generates a 14π-electron dianion. The NMR spectrum of the resulting dianion shows a diamagnetic ring current indicative of aromatic character, even though steric interactions among the internal hydrogens must prevent complete coplanarity. In contrast to the neutral [12]-annulene, which is thermally unstable above −50°C, the dianion remains stable at 30°C. The dianion of [16]-annulene has also been prepared and shows properties consistent with its being regarded as aromatic. It is consistent with the applicability of Huckel’s rule to charged, as well as neutral, conjugated planar cyclic structures.
Table 12.1 Hückel’s Rule Relationships for Charged Species
| Compound | π electrons |
|---|---|
| Aromatic species | |
| Cyclopropenium cation | 2 |
| Cyclopentadienide anion | 6 |
| Cycloheptatrienyl cation | 6 |
| Cyclooctatetraene dianion | 10 |
| Cyclononatetraenide anion | 10 |
| [12]-Annulene dianion | 14 |
| Anti-aromatic species | |
| Cyclopropenide anion | 4 |
| Cyclopentadienyl cation | 4 |
| Non-aromatic species | |
| Cycloheptatrienyl anion | 8 |
Leave a Reply