The original explanation of Woodward and Hoffmann (1965, 1970) involved generating a so-called ‘orbital correlation diagram’ for the reaction under consideration, and then carrying out the reaction in such a manner that the symmetries of the reactant and product orbitals matched exactly. This enables a correlation diagram for the reaction to be constructed, according to the following rules: no two orbitals of the same symmetry can cross during the reaction, whilst orbitals of different symmetry can cross. The favoured pathway is the one that results in a product of the same electronic excitation as the reactant. A pathway, that results in the product in a higher electronic state than the reactant is said to be ‘forbidden’.
These correlation diagrams can be generalized for any electrocyclic reaction with appropriate symmetry. However, correlation diagrams are less readily applied for reactions with no symmetry. Dewar and Zimmerman independently noticed that the ‘topological’ properties of these correlation diagrams are very similar to those obtained using, for example, the Huckel theory for aromatic molecules. For example, the diagram for the electrocyclic conversion of hexatriene to cyclohexadiene is remarkably similar at the transition state to the ground state orbitals of benzene.
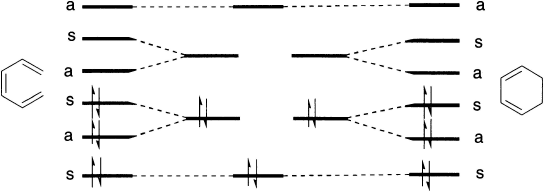
Such an approach, whilst theoretically rigorous, is not readily applicable to the majority of more complex reactions. Two much simpler methods have been outlined.
- The Overlap of Frontier Orbitals (HOMOs and LUMOs)
- The Concept of Transition State Aromaticity
In a practical sense, the first of these is the most easily remembered and applied. Although it may not seem obvious, this rule is actually derived from the original Woodward- Hoffmann approach. The frontier molecular method in which only the interaction between highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) is considered is the simplest and is capable of explaining the stereochemistry of almost all pericyclic reactions.
Leave a Reply