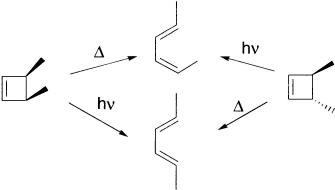
An electrocyclic reaction is an intramolecular reaction that involves the concerted formation of a sigma bond between the two ends of a linear conjugated π (pi) system, or the reverse reaction in which the sigma bond in a cyclic reactant is broken to produce a linear conjugated π system. The product in cyclic compound has one less π bond than the reactant acyclic system.

The reactions are stereospecific. For example, cis-3,4-dimethylcyclobutene gives solely Z,E-2,4-hexadiene, which is not the thermodynamically most stable isomer.
An electrocyclic reaction is completely stereoselective. For example, when 2E,4Z,6E-octatriene undergoes an electrocyclic reaction under thermal conditions, only the cis product is formed; when 2E,4Z,6Z-octatriene undergoes an electrocyclic reaction under thermal conditions, only the trans product is formed.

However, when the reactions are carried out under photochemical conditions, the products have the opposite configuration.

Electrocyclic reactions are reversible. The cyclic compound is favoured for electrocyclic reactions that form six-member rings, whereas the open-chain compound is favoured for electrocyclic reactions that form four-member rings because of the angle strain associated with four-member rings.
Electrocyclic reactions are completely stereoselective and stereospecific. All the electrocyclic reactions are accounted for by Frontier Molecular Orbital (FMO) approach by looking only at the symmetries of the two outermost lobes of the polyene. Thus, the inner lobes may not be shown. The lobes of like sign can be either on the same side or on opposite sides of the molecule. For bond formation, the outermost lobes must rotate—a positive lobe overlapping a positive lobe or a negative lobe overlapping a negative lobe.
Leave a Reply