You can often loosen a tight metal jar lid by holding it under a stream of hot water. Both the metal of the lid and the glass of the jar expand as the hot water adds energy to their atoms. (With the added energy, the atoms can move a bit farther from one another than usual, against the spring-like interatomic forces that hold every solid together.) However, because the atoms in the metal move farther apart than those in the glass, the lid expands more than the jar and thus is loosened.
Such thermal expansion of materials with an increase in temperature must be anticipated in many common situations. When a bridge is subject to large seasonal changes in temperature, for example, sections of the bridge are separated by expansion slots so that the sections have room to expand on hot days without the bridge buckling. When a dental cavity is filled, the filling material must have the same thermal expansion properties as the surrounding tooth; otherwise, consuming cold ice cream and then hot coffee would be very painful. When the Concorde aircraft (Fig. 18-9) was built, the design had to allow for the thermal expansion of the fuselage during supersonic flight because of frictional heating by the passing air.
The thermal expansion properties of some materials can be put to common use. Thermometers and thermostats may be based on the differences in expansion between the components of a bimetal strip (Fig. 18-10). Also, the familiar liquid-in-glass thermometers are based on the fact that liquids such as mercury and alcohol expand to a different (greater) extent than their glass containers.
Linear Expansion
If the temperature of a metal rod of length L is raised by an amount ΔT, its length is found to increase by an amount

in which α is a constant called the coefficient of linear expansion. The coefficient α has the unit “per degree” or “per kelvin” and depends on the material. Although α varies somewhat with temperature, for most practical purposes it can be taken as constant for a particular material. Table 18-2 shows some coefficients of linear expansion. Note that the unit C° there could be replaced with the unit K.
The thermal expansion of a solid is like photographic enlargement except it is in three dimensions. Figure 18-11b shows the (exaggerated) thermal expansion of a steel ruler. Equation 18-9 applies to every linear dimension of the ruler, including its edge, thickness, diagonals, and the diameters of the circle etched on it and the circular hole cut in it. If the disk cut from that hole originally fits snugly in the hole, it will continue to fit snugly if it undergoes the same temperature increase as the ruler.
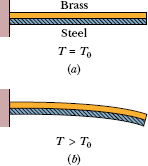
Fig. 18-10 (a) A bimetal strip, consisting of a strip of brass and a strip of steel welded together, at temperature T0. (b) The strip bends as shown at temperatures above this reference temperature. Below the reference temperature the strip bends the other way. Many thermostats operate on this principle, making and breaking an electrical contact as the temperature rises and falls.
Volume Expansion
If all dimensions of a solid expand with temperature, the volume of that solid must also expand. For liquids, volume expansion is the only meaningful expansion parameter. If the temperature of a solid or liquid whose volume is V is increased by an amount ΔT, the increase in volume is found to be

where β is the coefficient of volume expansion of the solid or liquid. The coefficients of volume expansion and linear expansion for a solid are related by
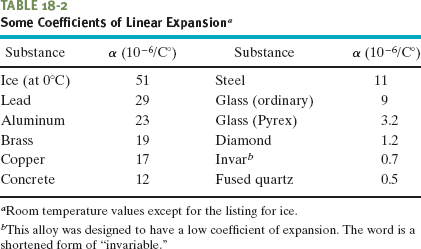
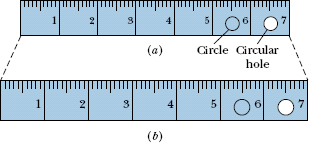
Fig. 18-11 The same steel ruler at two different temperatures. When it expands, the scale, the numbers, the thickness, and the diameters of the circle and circular hole are all increased by the same factor. (The expansion has been exaggerated for clarity.)

The most common liquid, water, does not behave like other liquids. Above about 4°C, water expands as the temperature rises, as we would expect. Between 0 and about 4°C, however, water contracts with increasing temperature. Thus, at about 4°C, the density of water passes through a maximum. At all other temperatures, the density of water is less than this maximum value.
This behavior of water is the reason lakes freeze from the top down rather than from the bottom up. As water on the surface is cooled from, say, 10°C toward the freezing point, it becomes denser (“heavier”) than lower water and sinks to the bottom. Below 4°C, however, further cooling makes the water then on the surface less dense (“lighter”) than the lower water, so it stays on the surface until it freezes. Thus the surface freezes while the lower water is still liquid. If lakes froze from the bottom up, the ice so formed would tend not to melt completely during the summer, because it would be insulated by the water above. After a few years, many bodies of open water in the temperate zones of Earth would be frozen solid all year round—and aquatic life could not exist.
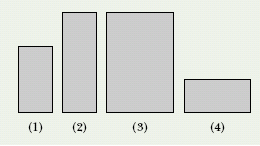
CHECKPOINT 2 The figure here shows four rectangular metal plates, with sides of L, 2L, or 3L. They are all made of the same material, and their temperature is to be increased by the same amount. Rank the plates according to the expected increase in (a) their vertical heights and (b) their areas, greatest first.
On a hot day in Las Vegas, an oil trucker loaded 37 000 L of diesel fuel. He encountered cold weather on the way to Payson, Utah, where the temperature was 23.0 K lower than in Las Vegas, and where he delivered his entire load. How many liters did he deliver? The coefficient of volume expansion for diesel fuel is 9.50 × 10−4/C°, and the coefficient of linear expansion for his steel truck tank is 11 × 10−6/C°.
Solution: The Key Idea here is that the volume of the diesel fuel depends directly on the temperature. Thus, because the temperature decreased, the volume of the fuel did also. From Eq. 18-10, the volume change is
Thus, the amount delivered was
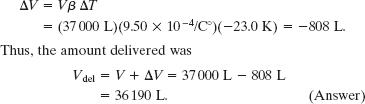
Note that the thermal expansion of the steel tank has nothing to do with the problem. Question: Who paid for the “missing” diesel fuel?
Leave a Reply