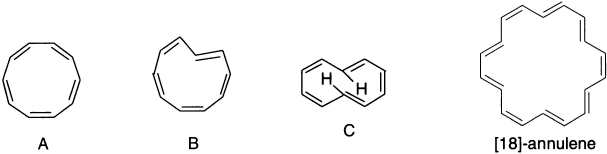
The term annulene was coined to refer to the completely conjugated monocyclic polyenes. Conjugated monocyclic polyenes (CnHn), in which n is greater than or equal to 10, are usually called annulenes. The number in brackets tells us how many carbon atoms there are in the ring. The synthesis of annulenes has been extended well beyond the first two members of the series [4]-annulene (cyclobutadiene) and [6]-annulene (benzene). Considering the properties of members of the annulene series can test the generality of the Hückel rule. The annulenes prepared have n = 12, 14, 16, 18, 20, 24 and 30. Of these only [14]-, [18]- and [30]-annulenes are (4n + 2) π electron molecules, whereas the rest are (4n)π molecules.
The smallest annulene of the (4n + 2) π type is [10]-annulene. Van Tamelen et al. (1971) have synthesized it by photolysis of trans-9,10-dihydronaphthalene. There are three possible isomers of [10]-annulene: (A) all-cis, (B) mono-trans and (C) cis-trans-cis-cis-trans. In case of isomer A, where all five double bonds are cis and planar, each internal angle would be 144°. Since a normal double bond has angles of 120°, this would be far from ideal. So this compound cannot adopt a planar conformation and, therefore, is not aromatic even though it has 10π electrons. By contrast, [18]-annulene with 18π electrons (n = 4) adopts a planar conformation and is aromatic. NMR spectrum indicates that it has a ring current characteristic of aromatic compounds. The resonance energy from combustion data has been estimated to be 100 kcal/mol.
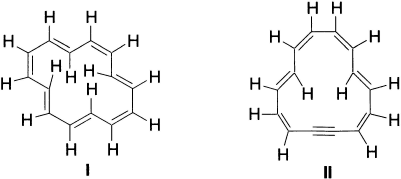
[14]-Annulene (I) follows the Huckel rule (14π electrons), is non-aromatic and unstable. This is attributed to the steric hindrance of the four internal hydrogens, which twist it out of planarity. However, monodehydro[14]-annulene (II) containing 14π electrons is planar and aromatic.
The smallest member, cyclobutadiene, was the object of attempted synthesis for many years. The first success was achieved when cyclobutadiene released from a stable iron complex was trapped with various reagents. Dehalogenation of trans-3,4-dibromocyclobutene was shown to generate a species with the same reactivity. Various trapping agents react with cyclobutadiene to give Diels-Alder adducts. In the absence of trapping agents, a characteristic dimer is produced. This dimerization is an extremely fast reaction and limits the lifetime of cyclobutadiene, except at very low temperatures.
Cyclobutadiene can also be prepared by photolysis of several different precursors at very low temperature in solid inert gases. These methods provide cyclobutadiene in a form that is appropriate for spectroscopic study. Under these conditions, cyclobutadiene begins to dimerize at around 35 K. Whereas simple HMO theory assumes a square geometry for cyclobutadiene, most molecular orbital methods predict a rectangular structure as the minimum-energy geometry. With very high-level calculations, good agreement is obtained between the calculated minimum-energy structure, which is rectangular, and observed spectroscopic properties.
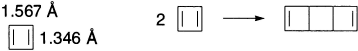
The rectangular structure is calculated to be strongly destabilized (antiaromatic) with respect to a polyene model. For example, cyclobutadiene is found to have negative resonance energy of −54.7 kcal/mol, relative to 1,3-butadiene. In addition, 30.7 kcal of strain is found, giving a total destabilization of 85.4 kcal/mol. A number of alkyl-substituted cyclobutadienes have been prepared by related methods. Increasing alkyl substitution enhances the stability of the compounds. The tetra-t-butyl derivative is stable up to at least 150°C but is very reactive toward oxygen. This reactivity reflects the high energy of the HOMO. The chemical behaviour of the cyclobutadienes as a group is in excellent accord with that expected from the theoretical picture of the structure of these compounds.
[6]-Annulene is benzene. Its properties are so familiar to students of organic chemistry that not much need be said here. It is the parent compound of a vast series of derivatives. The benzene ring shows exceptional stability, both in a thermodynamic sense and in terms of its diminished reactivity in comparison with conjugated polyenes. As was discussed earlier, stabilization on the order of 30 kcal/mol is found by thermodynamic comparisons. Benzene is much less reactive toward electrophiles than are conjugated polyenes. This is in line with the HOMO of benzene being of lower energy than the HOMO of a conjugated polyene.
The next higher annulene, cyclooctatetraene, is nonaromatic. The bond lengths around the ring alternate as expected for a polyene. The C=C bonds are 1.33 Å and the C–C bonds are 1.462 Å in length. Thermodynamic data provide no evidence of any special stability. Cyclooctatetraene is readily isolable, and its chemical reactivity is normal for a polyene. Structure determination shows that the molecule is tub-shaped and, therefore, is not a planar system to which the Huckel rule applies. There have been both experimental and theoretical studies aimed at trying to estimate the stability of the planar form of cyclooctatetraene. The results indicate that the completely delocalized D8h structure is about 4.1 kcal higher in energy than the conjugated planar D4h structure, suggesting that delocalization leads to destabilization.
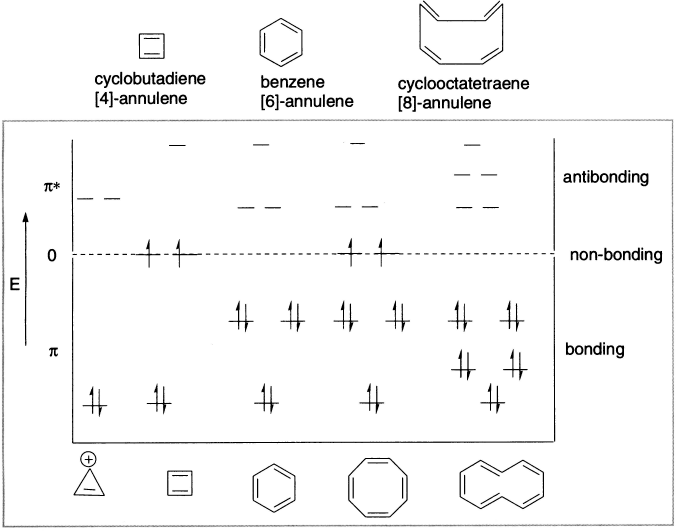
Figure 12.2 HMO π-energy level diagram of annulenes
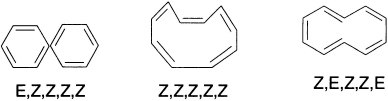
Larger annulenes permit the incorporation of trans double bonds into the rings. Beginning with [10]-annulene, stereoisomeric structures are possible. According to the Huckel rule, [10]-annulene should possess aromatic stabilization if it were planar. However, all the isomeric cyclodeca-l,3,5,7,9-pentaenes suffer serious steric strain that prevents the planar geometry from being adopted. The Z,E,Z,Z,E-isomer, which has minimal bond-angle strain, suffers a severe nonbonded repulsion between the two internal hydrogens.
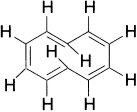
The Z,Z,Z,Z,Z-isomer is required by geometry to have bond angles of 144° to maintain planarity and would, therefore, be enormously destabilized by distortion of the normal trigonal bond angle. The most stable structure is a twisted form of the E,Z,Z,Z,Z-isomer. Molecular orbital calculations suggest an aromatic stabilization of almost 18 kcal for a conformation of the E,Z,Z,Z,Z-isomer in which the inner hydrogens are twisted out of the plane by about 20°, but other calculations point to a polyene structure.
Experimental studies have indicated that all of the isomers prepared to date are quite reactive, but whether the most stable isomer has been observed is uncertain. Two of the isomeric [10]-annulenes, as well as other products, are formed by photolysis of cis-9,10-dihydronaphthalene. Neither compound exhibits properties that would suggest aromaticity. The NMR spectra are consistent with polyene structures. Both compounds are thermally unstable and revert back to dihydronaphthalenes. It appears that [10]-annulene is sufficiently distorted from planarity that little stabilization is achieved.
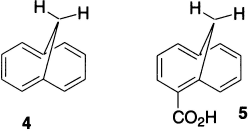
A number of structures have been prepared that do not have the steric problems associated with the cyclodeca-1,3,5,7,9-pentaenes. In compound 4, the steric problem is avoided with only a slight loss of planarity in the π system. The NMR spectrum of this compound shows a diamagnetic ring current of the type expected in an aromatic system. Thus, its carboxylic acid derivative 5 is also prepared.
Most molecular orbital methods find a bond alternation pattern in the minimum-energy structure, but calculations that include electron correlation lead to a delocalized minimum-energy structure. Thus, although the π system in 4 is not completely planar, it appears to be sufficiently close, providing a delocalized 10-electron π system. Resonance energy of 17.2 kcal has been obtained on the basis of an experimental heat of hydrogenation.
[12]-Annulene is a very unstable compound that undergoes cyclization to bicyclic isomers and can be kept only at very low temperature. The NMR spectrum has been studied at low temperature. Besides indicating the double bond geometry shown in the structure drawn here, the spectrum reveals a paramagnetic ring current, the opposite to what is observed for aromatic systems. This feature is quite characteristic of the [4n] annulenes and has been useful in characterizing the aromaticity or lack of it in annulenes.
[14]-Annulene was first prepared in 1960. Its NMR spectrum has been investigated and shows that two stereoisomers are in equilibrium.
The spectrum also reveals a significant diamagnetic (aromatic) ring current. The signals of the internal hydrogens (C-3, C-6, C-10 and C-13) are very far upfield (δ = 0.61 ppm). The interconversion of the two forms involves a configurational change from E to Z at least one double bond. The activation energy for this process is only about 10 kcal/mol. The crystal structure for [14]-annulene shows the Z,E,E,Z,E,Z,E-form to be present in the solid. The bond lengths around the ring range from 1.35 to 1.41Å but do not show the alternating pattern of short and long bonds expected for a localized polyene. There is some distortion from planarity, especially at carbon atoms 3, 6, 10 and 13, which is caused by nonbonded repulsions between the internal hydrogens. A 14-electron π system can be generated in circumstances in which the steric problem associated with the internal hydrogens of [14]-annulene is avoided.
Several derivatives of this ring system have been synthesized. These compounds exhibit properties indicating that the conjugated system is aromatic. They exhibit NMR shifts characteristic of a diamagnetic ring current. Typical aromatic substitution reactions can be carried out. An X-ray crystal structure shows that the bond lengths are in the aromatic range (1.39–1.40Å), and there is no strong alternation around the ring. The peripheral atoms are not precisely planar, but the maximum deviation from the average plane is only 0.23Å. The dimethyl derivative is essentially planar, with bond lengths between 1.38 and 1.40Å.
The Huckel rule predicts non-aromaticity for [16]-annulene. The compound has been synthesized and thoroughly characterized. The bond lengths show significant alternation (C=C, 1.34Å; C–C, 1.46Å), and the molecule is less planar than [14]-annulene. These structural data are consistent with regarding [16]-annulene as being non-aromatic.
[18]-Annulene offers a particularly significant test of the Huckel rule. The internal cavity in [18]-annulene is large enough to minimize steric interactions between the internal hydrogens in a geometry that is free of angle strain. Most molecular orbital calculations find the delocalized structure to be more stable than the polyene.
The properties of [18]-annulene are consistent with its being aromatic. The X-ray crystal structure shows the molecule to be close to planarity, with the maximum deviation from the plane being 0.085Å. The bond lengths are in the range 1.385–1.405Å. The NMR spectrum is indicative of an aromatic ring current. At about −60°C, the protons outside the ring of [18]-annulene are strongly deshielded (δ 9.3) and those inside are strongly shielded (δ −3.0, that is, more shielded than TMS). Demonstration of such a ring current is good evidence for planarity and aromaticity, at least at low temperature. As the temperature is raised, the signals broaden because of slow interchanges in ring conformations. At about 110°C, a single averaged peak appears at approximately δ 5.3 because of rapid interchanges in ring conformations, to give an averaged chemical shift. The chemical reactivity of the molecule would also justify its classification as aromatic. The bond lengths are 1.39–1.42Å, and a stabilization of 18 kcal/mol is indicated.
The synthesis of annulenes has been carried forward to larger rings as well. [20]-Annulene, [22]-annulene and [24]-annulene have all been reported. The NMR spectrum of [22]-annulene is consistent with regarding the molecule as aromatic, whereas those of the [20] and [24] analogs are not. In each case, there is some uncertainty as to the preferred conformation in solution, and the NMR spectra are temperature-dependent. Although the properties of these molecules have not been studied as completely as those of the smaller systems, they are consistent with the predictions of the Huckel rule.
Both clever synthesis and energetic processes leading to stable compounds have provided other examples of structures for which aromaticity might be important. Kekulene was synthesized in 1978. How aromatic is this substance? Both by energy and magnetic criteria, it appears that it is primarily benzenoid in character. Its energy is close to that expected from isodesmic reactions summing smaller aromatic components. Magnetic criteria, too, indicate that it is similar to the smaller polycyclic benzenoid hydrocarbons, such as phenanthrene and anthracene.
It has been pointed out that a different array of atomic orbitals might be conceived of in large conjugated rings. The array, called a Mobius twist results in there being one point in the ring at which the atomic orbitals would show a phase discontinuity. If the ring were sufficiently large that the twists between individual orbitals were small, such a system would not necessarily be less stable than the normal array of atomic orbitals. This same analysis points out that in such an array the Huckel rule is reversed and aromaticity is predicted for the 4n π-electron systems.
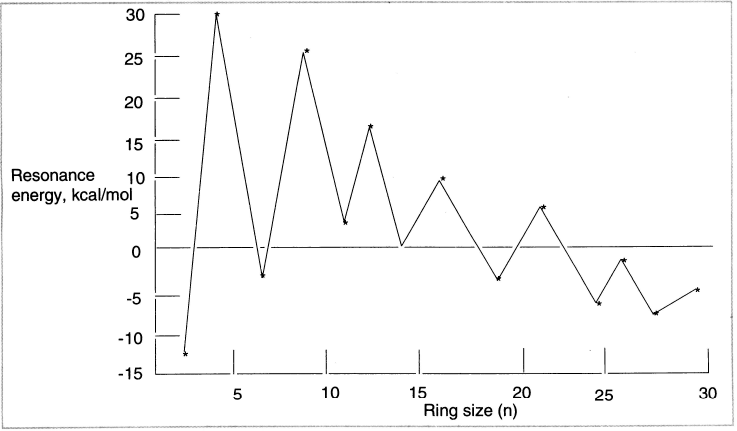
Figure 12.3 Change in resonance energies for annulenes
Chemical reactivity data suggest that larger rings may be less aromatic than the smaller ones. If we take a large delocalization energy as a measure of aromaticity, then the distribution between what is aromatic and what is anti-aromatic becomes smaller and smaller with increasing ring size, and for a ring of around 26π centres, even a member of 4n + 2 series no longer is calculated to be more stable than its acyclic analogues.
Leave a Reply