In a cycloaddition reaction, the orbitals of one molecule must overlap with the orbitals of the second molecule. Therefore, the frontier molecular orbitals of both reactants must be evaluated to determine the outcome of the reaction. Since the new σ bonds in the product are formed by donation of electron density from one reactant to the other, we must consider the HOMO of one of the molecules and the LUMO of the other, because only an empty orbital can accept electrons. It does not matter which reacting molecule’s HOMO is used. It is required only that we use the HOMO of the one and the LUMO of the other.
There are two modes of orbital overlap for the simultaneous formation of two σ bonds: suprafacial and antarafacial. Bond formation is suprafacial if both σ bonds form from the same side of the π system. Bond formation is antarafacial if the two σ bonds form from opposite sides of the π system. Suprafacial bond formation is similar to syn addition whereas antarafacial bond formation resembles anti addition.
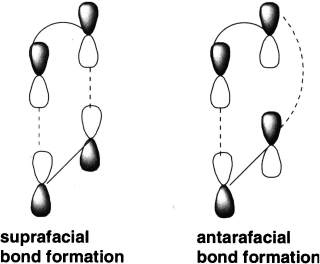
A cycloaddition reaction that forms a four-, five-, or six-member ring must involve suprafacial bond formation. The geometric constraints of these small rings make the antarafacial approach highly unlikely, even if it is symmetry-allowed (the overlapping orbitals are in phase). Antarafacial bond formation is more likely in cycloaddition reactions that form larger rings.
Frontier orbital analysis of a [4+2] cycloaddition reaction shows that overlap of in-phase orbitals to form the two new σ bonds requires suprafacial orbital overlap. This is true whether we use the HOMO of the alkene (a system with one π bond) and the LUMO of the diene (a system with two conjugate π bonds) or the LUMO of the alkene and the HOMO of the diene. Now we can understand why Diels-Alder reactions occur with relative ease. It is clear that in both combinations, there are bonding interactions at the termini. The [π2s+π4s] addition is thus thermally or symmetry allowed. Since suprafacial–suprafacial and antarafacial–antarafacial combinations give the same prediction, [π4a + π2a] addition is also thermally allowed. In photochemical reaction, the reverse is true, that is, [π2a + π4s] or [π4a + π2s] addition takes place.
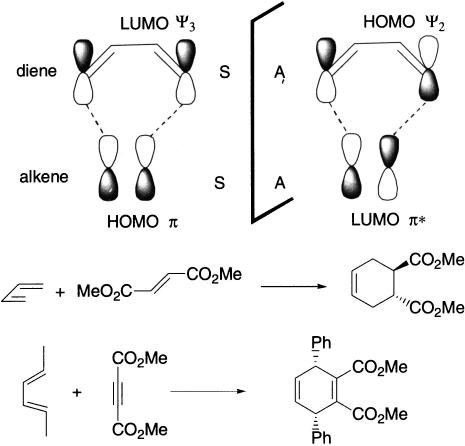
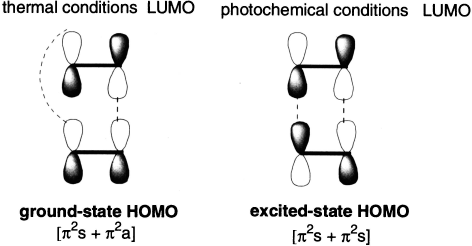
A [2+2] cycloaddition reaction does not occur under thermal conditions but does take place under photochemical conditions.
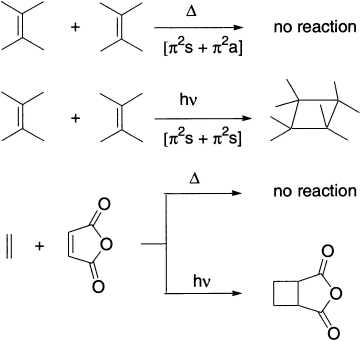
The reason for this is apparent from an examination of the frontier molecular orbitals. Under thermal conditions, suprafacial overlap is not symmetry-allowed (the overlapping orbitals are out of phase). Antarafacial overlap is symmetry-allowed, but is not possible because of the small size of the ring. Under photochemical conditions, the reaction can take place because the excited-state HOMO has symmetry opposite that of the ground state HOMO. Therefore, overlap of the excited-state HOMO of one alkene with the LUMO of the second alkene involves symmetry-allowed suprafacial bond formation.
Notice that in the photochemical reaction, only one of the reactants is in an excited state. Owing to the very short lifetimes of excited states, there is little likelihood that two excited states will find one another to interact.
Similar treatment of the cycloaddition of two butadiene molecules – an 8π electron (m + n = 8) system shows that [π4s + π4a] addition is thermally allowed and [π4s + π4s] is photochemically allowed. A [6+2] addition behaves in the same fashion. A [6 + 4] cycloaddition behaves exactly in the same way as the [4 + 2] addition.
Woodward–Hoffmann rules In a thermal pericyclic reaction the total number of (4q + 2)s and (4r)a components must be odd. A component is a bond or orbital taking part in a pericyclic reaction as a single unit. A double bond is a π2 component. The number 2 is the most important part of this designation and simply refers to the number of electrons. The prefix π tells us the type of electrons. A component may have any number of electrons (a diene is a π4 component) but may not have mixtures of π and σ electrons. Now look back at the rule. Those mysterious designations, (4q+2) and (4r), simply refer to the number of electrons in the component where q and r are integers. An alkene is a π2 component and so it is of the (4q+2) kind, a diene is a π4 so is of the (4r) kind.
A few relevant points emerge from the above discussion:
- For a two-component cycloaddition, the maximum number of modes of addition is 22 (for n components, it is 2n): (s,s), (a,s), (s,a) and (a,a).
- Only in the (s,s) mode of addition do the two π systems approach in parallel plane. In all other modes of addition, the components approach orthogonally.
- Configuration of groups at the two termini of a suprafacial component is retained, but that on a antarafacial component is inverted.
- For either m or n greater than 2, there are two modes of (s,s) additions giving endo– and exo-products.
Now what about the suffixes ‘s’ and ‘a’? The suffix ‘s’ stands for suprafacial and ‘a’ for antarafacial. A suprafacial component forms new bonds on the same face at both ends, whereas an antarafacial component forms new bonds on opposite faces at both ends. See how this works for the Diels-Alder reaction. Here is the routine.
- Draw the mechanism for the reaction (we shall choose a general one)
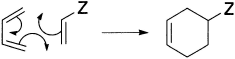
- Choose the components. All the bonds taking part in the mechanism must be included and no others
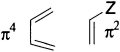
- Make a three-dimensional drawing of the way the components come together for the reaction, putting in orbitals at the ends of the components (only!)
- Join up the components where new bonds are to be formed. Coloured dotted lines are often used.
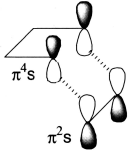
- Label each component s or a depending on whether new bonds are formed on the same or on opposite sides.
- Count the number of (4q+2)s and (4r)a components. If the total count is odd, the reaction is allowed. There is one (4q+2)s component (the alkene) and no (4r)a components, total = 1, so it is an allowed reaction.
- The cycloadditions that do occur thermally, for example, the Diels-Alder reaction, have (4n+2π) electrons in their ‘aromatic’ transition states.
- The cycloadditions that do not occur thermally, for example, the dimerization of alkenes and of dienes, has 4nπ electrons in their ‘anti-aromatic’ transition states.
Leave a Reply