There is a group of closely related rearrangements in which carbon migrates from carbon to nitrogen that may be represented generally as,
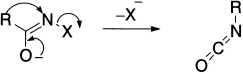
where R is an alkyl or aryl group and −X is a leaving group, which may be – Br (Hofmann rearrangement), ![]() (Curtius and Schmidt rearrangement) and – OCOR (Lossen rearrangement). In each case, if the migrating group is asymmetric, it retains its configuration.
(Curtius and Schmidt rearrangement) and – OCOR (Lossen rearrangement). In each case, if the migrating group is asymmetric, it retains its configuration.
Hofmann Rearrangement
The Hofmann rearrangement involves conversion of a carboxylic acid amide into an amine with loss of a carbon atom on treatment with aqueous sodium hypobromite (usually generated in situ from bromine and sodium hydroxide). Thus, it results in a shortening of the carbon chain by one atom and a change in the functional group from an amide to an amine. The Hofmann rearrangement occurs through a pathway similar to that for the Beckmann rearrangement.
The first step in the reaction sequence is the formation of the N-bromoamide, which then undergoes removal of the remaining proton on the nitrogen, the acidity of which is enhanced due to the presence of the electronegative bromine in addition to the acyl group. The deprotonated intermediate subsequently undergoes rearrangement by what is generally accepted to be a concerted mechanism, although a two-step process involving loss of bromide and formation of a nitrene would also be compatible with the data available.
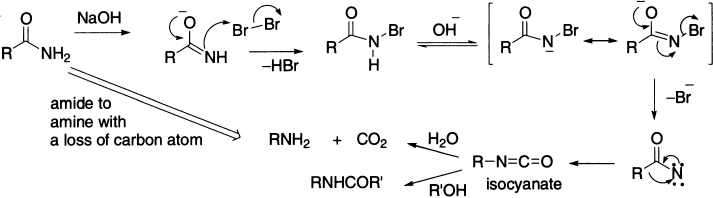
The acid amide is treated with sodium hypobromite or bromine in alkali to give N-bromoamide, which reacts with the base to give its conjugate base. Separation of halide ion from the conjugate base gives an electron-deficient species called nitrene within which rearrangement occurs (nitrene is high-energy–neutral species containing a sextet of electrons on nitrogen atom, which is isoelectronic with carbenes).
The rearrangement of nitrene to isocyanate is intramolecular and analogous to the conversion of an acyl carbene to a ketene. Intramolecular process has been established by isotopic labelling: when two amides are rearranged no cross-products are obtained. The isocyanate produced may be isolated in anhydrous conditions, but reaction is generally carried out in aqueous or alcoholic solution in which the isocyanate is converted into an amine or a urethane, respectively. Increase in the nucleophilicity of the migrating group facilitates the reaction.
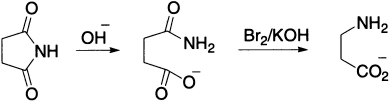
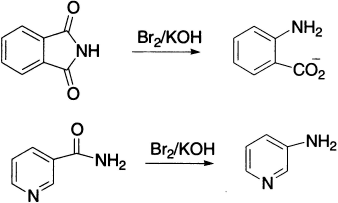
This rearrangement provides an efficient route for making both aliphatic and aromatic primary amines. Succinimide, on treatment with Br2 and aqueous KOH, yields about 45% ß–alanine.
Anthranilic acid may be obtained in 85% yield in a similar way from phthalimide. Nicotinamide gives ß-aminopyridine (65–70%) that cannot be obtained in good yield via the nitration of pyridine.
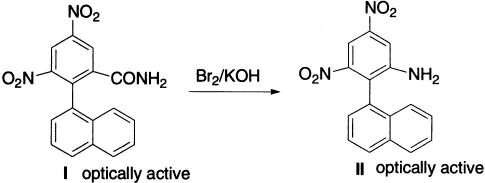
An optically active amide (I) gives an optically active amine (II), showing that reaction proceeds through a transition state in which the migratory group is partially bonded to both the migrating origin and the terminus.
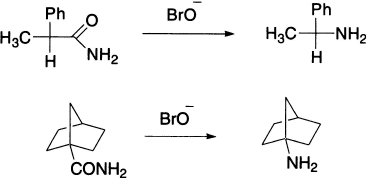
(+)-α-Phenylpropionamide, when subjected to sodium hypobromite, gives (−)-α-phenylethylamine with retention of configuration in the migrating group. Bicyclic amide undergoes Hofmann rearrangement with retention of configuration at the migrating atom, because rigidity of the ring system prohibits inversion of configuration.
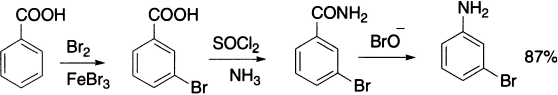
Meta-bromoaniline can be synthesized from benzoic acid involving Hofmann rearrangement.
α,β-Unsaturated amides give satisfactory yields of urethanes when treated with methanolic sodium hypochlorite. Hydrolysis of these urethanes lead directly to aldehydes, as would be expected, and, therefore, is best carried out in an acid medium.
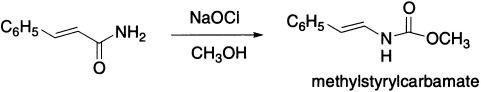
With α,β-acetylenic amides the Hofmann reaction leads to the formation of nitriles.
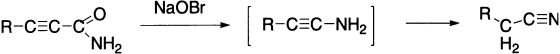
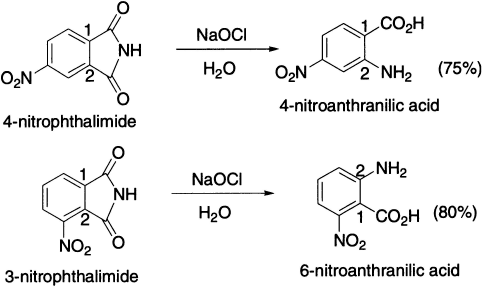
It is known that the hydrolysis of benzamides is facilitated by substituents that withdraw electrons from the amide linkage into the ring. It is, therefore, evident that in the Hofmann reaction of 4-nitrophthalimide, the nitro group, by withdrawing electrons at position 1, will cause preferential hydrolysis of the amide linkage at this point, with subsequent rearrangement at position 2.
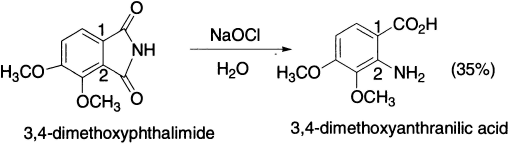
Furthermore, it is known that a methoxy group in the ortho-position to the amide linkage is much less effective in promoting hydrolysis than in the para-position. For example, in the Hofmann reaction of 3,4-dimethylphthalimide, hydrolysis of amide linkage should occur preferentially at 1-position.
Urea, when subjected to Hofmann reaction, yields 60% hydrazine.
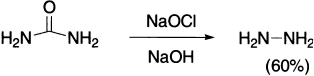
Curtius Degradation (Rearrangement)
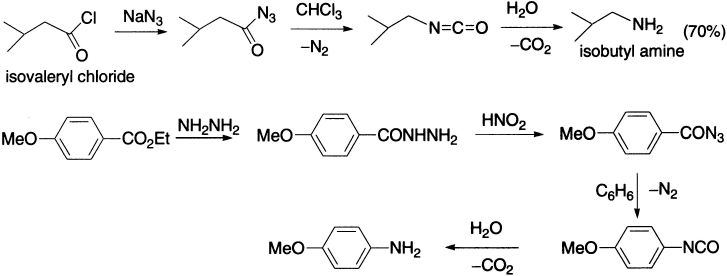
Curtius rearrangement involves pyrolysis of an acyl azide that expels molecular nitrogen and, at the same time, rearranges to an isocyanate (the azides may be made by nucleophilic substitution on an acyl chloride by sodium azide or by the reaction of acyl hydrazides with nitrous acid). Azides must be treated with caution as they may decompose explosively. The isocyanate may be isolated by carrying out the reaction in an aprotic solvent such as chloroform, but, generally, alcoholic solvent is used with which the isocyanate reacts to form a urethane.
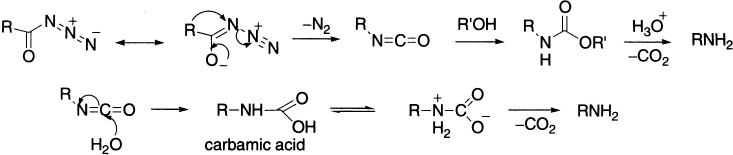
The addition of water to isocyanate initially results in a carbamic acid, which is unstable and decarboxylate to give amines. The amines formed contain one carbon atom less than the acyl azide substrates. It is due to this feature that the reaction is normally referred to as Curtius degradation rather than Curtius rearrangement
α-Hydroxy azides exhibit a unique behaviour; that is, the intermediate isocyanates lose cyanic acid and aldehydes or ketones are formed. γ-And δ-hydroxy azides revert to the lactone through loss of hydrazoic acid; this reaction occurs less readily when a nonpolar solvent is used in the rearrangement.
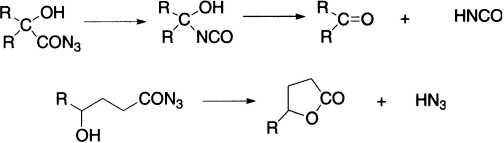
Halogenated azides undergo rearrangement in the usual manner to isocyanates, from which halo amines are obtained by hydrolysis. If the halogen is in the α-position, the resulting halogenated isocyanate is hydrolyzed to an aldehyde or ketone.
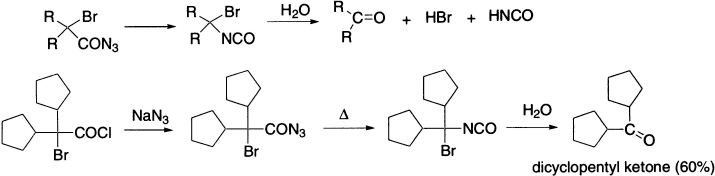
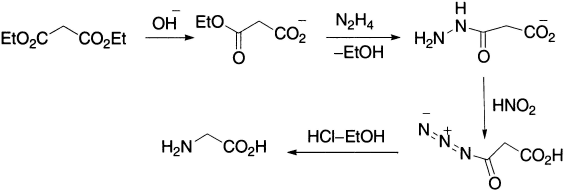
The Curtius rearrangement has been applied to the synthesis of α-amino acids:
Schmidt Reaction
Carboxylic acids react with hydrazoic acid in the presence of concentrated H2SO4 to give isocyanates directly. The acyl azide is normally not isolated, but allowed to decompose and rearrange in the reaction mixture itself.
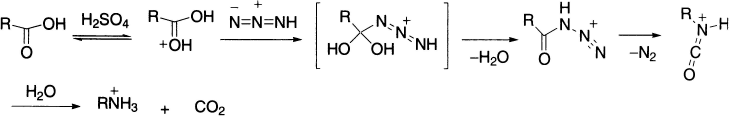
The Schmidt reaction is only applicable if the acid does not contain groups that are sensitive to concentrated sulphuric acid. Carbonyl compounds also undergo Schmidt reaction when treated with hydrazoic acid in sulphuric acid. Ketones produce amides, whereas aldehyde generally yields a mixture of the corresponding nitrile and N-formyl derivative.
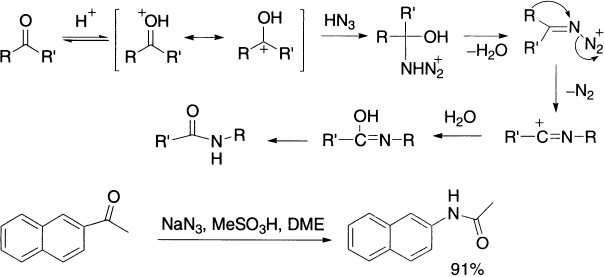
Lossen Rearrangement
The Lossen rearrangement differs from the Hofmann rearrangement only in the leaving group, which is carboxylate anion rather than bromide ion. The starting material is the ester of hydroxamic acid (RCONHOH) that is decomposed in presence of base. Hydroxamic acids exhibit tautomerism: the keto-form is termed hydroxamic form and the enol-form is called hydroximic acid. As the reaction is normally carried out in water, the process furnishes the amine directly.
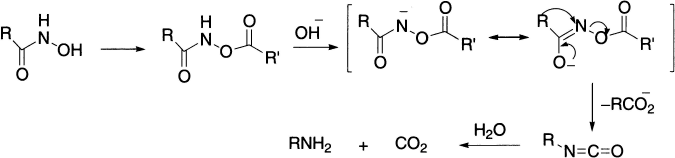
Since leaving group R’COO leaves as R’COO−, the rearrangement is facilitated by the presence of electron-withdrawing groups in meta– or para-positions. Of these four related processes, the Lossen rearrangement is the least useful in synthesis of amines because of unavailability of hydroxamic acids.
Leave a Reply