The rate-determining elimination step in an E1cB reaction is unimolecular, so you might imagine it would have a first-order rate equation. But, in fact, the rate is also dependent on the concentration of the base. This is because the unimolecular elimination involves a species–the anion–whose concentration is itself determined by the concentration of the base by the equilibrium we have just been discussing. Using the following general E1cB reaction, the concentration of the anion can be expressed as shown.
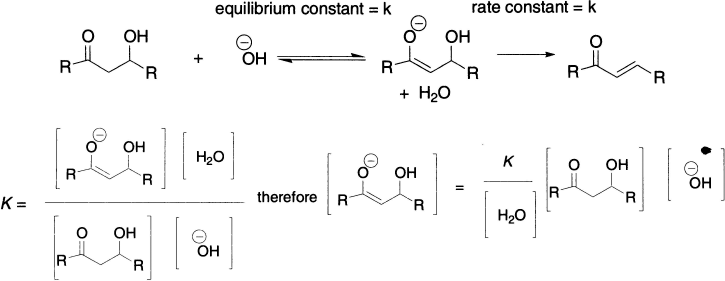
The rate is proportional to the concentration of the anion. We can simplify it further because the concentration of water is constant. Just because the base (hydroxide) appears in this rate equation, it does not mean that it is involved in the rate-determining step. Increasing the concentration of the base makes the reaction go faster by increasing the amount of anion available for elimination.
Leave a Reply