Haloforms undergo base-catalyzed α-elimination in two stages, the second of which is rate-determining with the formation of carbenes.
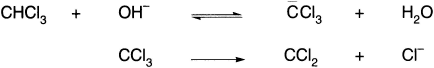
The carbanion intermediates owe their stability in part to the ability of all the halogens except fluorine to accommodate the negative charge in d orbitals. The resulting order of reactivity in the first step is CHI3 > CHBr3 > CHCl3 >> CHF3.
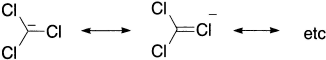
The stability of the carbenes is determined by the ability of the halogens to supply p-electrons to the electron-deficient carbon:

Since the +M effects of the halogens lie in the order F > Cl > Br > I, the ease of the second step in carbene formation is greatest when the haloform contains fluorine atoms.
4.2.1 Elimination When Nucleophile Attacks Hydrogen
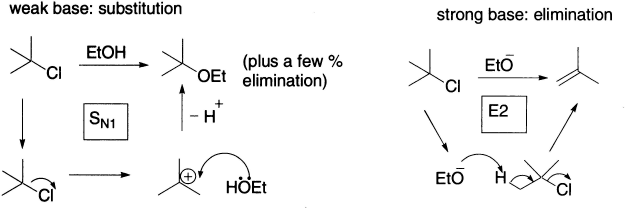
The elimination reaction of t-butyl bromide happens because the nucleophile is acting here as a base. You know that there is some correlation between basicity and nucleophilicity; strong bases are usually good nucleophiles. But being a good nucleophile doesn’t get hydroxide anywhere in the substitution reaction, because it does not appear in the first-order rate equation. Being a good base, however, does get it somewhere in the elimination reaction, because hydroxide is involved in the rate-determining step of the elimination, and so it appears in the rate equation.

Hydroxide behaves as a base because it attacks the hydrogen atom instead of the carbon atom it would attack in a substitution reaction. The hydrogen atom is not acidic, but proton removal can occur because bromide is a good leaving group. As the hydroxide ion attacks, the bromide is forced to leave, taking with it the negative charge. Two molecules, t-butyl bromide and hydroxide are involved in the rate-determining step of the reaction. This means that the concentrations of both appear in the rate equation, which is therefore of the second-order, and this mechanism for elimination is termed E2, which stands for elimination, bimolecular.
Rate = k2 [t-BuBr] [HO−]
Now let us look at another sort of elimination. We can approach it again by thinking about an SN1 substitution reaction.
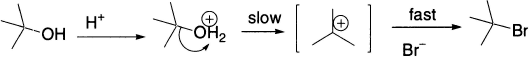
Bromide, the nucleophile, is not involved in the rate-determining step; so, we know that the rate of the reaction will be independent of the concentration of Br−. But what happens if we use an acid whose counter ion is such a weak nucleophile that it doesn’t even attack the carbon of the carbocation? Here is an example: t-butanol in sulphuric acid does not undergo substitution, but undergoes elimination instead.
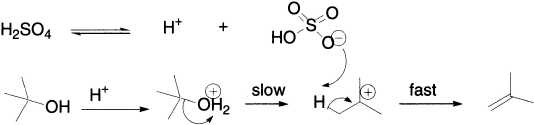
Now, the HSO4− is not involved in the rate-determining step. HSO4− is not at all nucleophilic and only behaves as a base (that is, it removes a proton) because it is even feebler as a nucleophile. The rate equation will not involve the concentration of HSO4−, and the rate-determinining step is the same as that in the SN1 reaction. The mechanism of unimolecular loss of water from the protonated t-BuOH is therefore called E1.
We will shortly come back to these two mechanisms for elimination, plus a third, but first we need to answer the question: when does a nucleophile start behaving as a base?
4.2.2 Nucleophile Effects on Elimination and Substitution
Basicity The molecule-bearing leaving groups are attacked at two distinct electrophilic sites: the carbon to which the leaving group is attached and the hydrogen atoms on the carbon adjacent to the leaving group. Attack at carbon leads to substitution; attack at hydrogen leads to elimination. Because strong bases attack protons, it is generally true that the more basic the nucleophile, the more likely that elimination is going to replace substitution as the main reaction of an alkyl halide.
Here is an example of this idea at work.
Size For a nucleophile, attacking a carbon atom means squeezing past its substituents; even for unhindered primary alkyl halides there is still one alkyl group attached. This is one of the reasons that SN2 is so slow on hindered alkyl halides; the nucleophile has difficulty getting to the reactive centre. Getting at a more exposed hydrogen atom in an elimination reaction is much easier, and this means that as soon as we start using hard, basic nucleophiles that are also bulky, elimination is preferred over substitution, even for primary alkyl halides. One of the best bases for promoting elimination and avoiding substitution is potassium t-butoxide. The large alkyl substitution makes it hard for the negatively charged oxygen to attack carbon in a substitution reaction, but it has no problem attacking hydrogen.
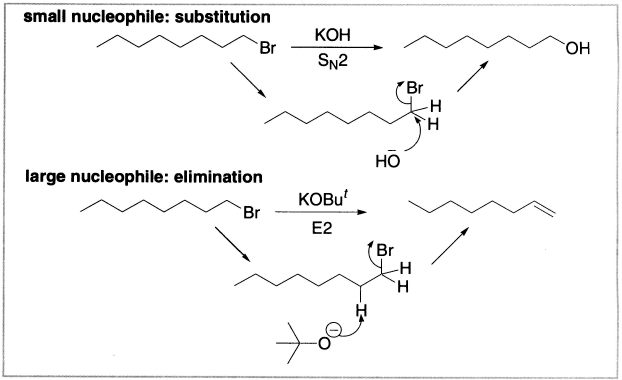
Figure 4.1 Elimination and substitution due to size
Temperature Temperature plays an important role in deciding whether a reaction is elimination or a substitution. In elimination, two molecules become three. In a substitution, two molecules form two new molecules. The two reactions differ therefore in the change in entropy during the reaction: ΔS is greater for elimination than for substitution.
ΔG = ΔH – TΔS
This equation says that a reaction in which ΔS is positive is more exothermic at a higher temperature. Eliminations should therefore be favoured at high temperature, and this is indeed the case. Most of the eliminations are conducted at room temperature or above.
These effects can be summarized as:
- Nucleophiles that are strong bases favour elimination over substitution
- Nucleophiles (or bases) that are bulky favour elimination over substitution
- High temperatures favour elimination over substitution

Leave a Reply