Reactions in which a molecule of two atoms or groups is removed from a compound and which are not replaced by other atoms or groups are referred to as elimination. It belongs to one of the important class of organic reactions. The atoms or groups can be bound to carbon atoms and/or to heteroatoms in the substrate. These atoms can be sp3 or sp2 hybridized.
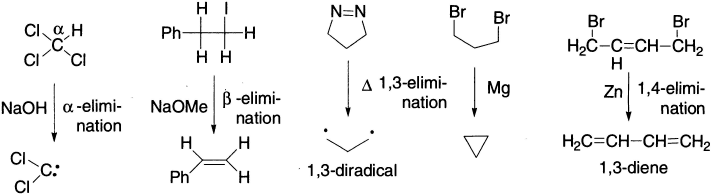
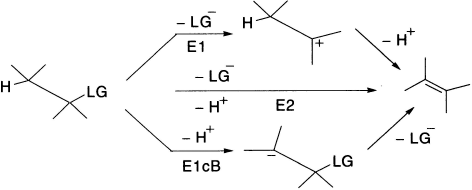
If eliminating atoms or groups are geminal, their removal is an α-elimination, which is used for generating the reactive intermediate called carbene. If they are vicinal, it is a β-elimination, the most common one. If they are separated from each other by n atoms, their removal is called 1,n-elimination, that is, 1,3-, 1,4-elimination, and so on. β-Elimination reactions can occur by three distinct limiting mechanisms; of these E2 and E1 are the most common; E1cB is less common and it operates only when the β-hydrogen is appreciably acidic.
Common substrates for elimination are alkyl and cycloalkyl halides, vic-dihalides, alcohols, ethers, esters, tetraalkylammonium halides and hydroxides, tetraalkylphosphonium halides and hydroxides, sulphonium halides and hydroxides, alkyldiazonium halides, aldoximes and ketoximes. Common leaving groups are H2O, ROH, RCOO−, NR3, PR3, SR2, N2, X−, etc.
Substitution and Elimination
Substitution reactions of t-butyl halides follow the SN1 mechanism. In other words, the rate-determining step of their substitution reactions is unimolecular, which involves only the alkyl halide. And this means that, no matter what the nucleophile is, the reaction goes at the same rate. One can not speed up the SN1 reaction by using hydroxide instead of water, or even by increasing the concentration of hydroxide.
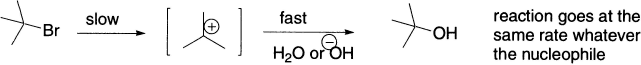
If one does the substitution reaction with a solution of sodium hydroxide, the reaction stops being a substitution, and an alkene is formed instead. Overall, HBr has been lost from the alkyl halide, and the reaction is called elimination.
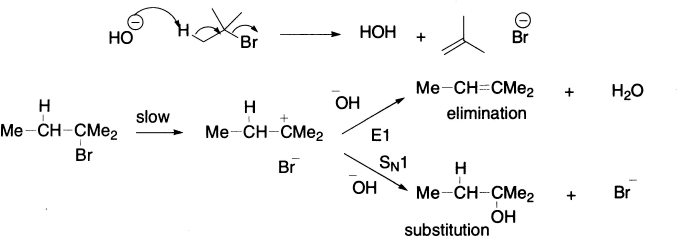
It might be expected that any structural features in the starting material that serve to stabilize the developing double bond would promote elimination at the expense of substitution. For example, under the same reaction condition, CH3–CH2−Br yields only 1% of alkene, whereas C6H5CH2–CH2–Br is found to yield 99% alkene. Another significant factor is the size of the base/nucleophile: the larger this is, the greater the proportion of elimination.
In this lesson, we will talk about the mechanisms of elimination reactions; as in the case of substitution, there is more than one mechanism for eliminations. In most of the organic eliminations, one of the eliminated groups is a hydrogen atom (usually H+) and the other is a leaving group (LG). We will compare eliminations with substitution; either reaction can happen from almost identical starting materials, and you will learn how to predict which is the more likely.
Leave a Reply